Paper title - Health Effects and Geochemistry of Arsenic and
advertisement

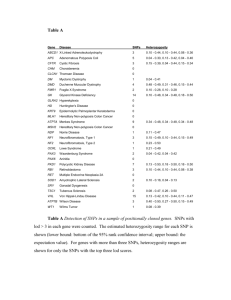
Gene-Environment Interactions between Arsenic Exposure from Drinking Water and Genetic Susceptibility in Cardiovascular Disease Risk and Carotid Artery Intima-Media Thickness in Bangladesh F. Wu, M.L. Liu, X. Cheng, J.Y. Jiang, Y. Chen New York University, New York, NY, USA F. Parvez, V. Slavkovich, D. Levy, J.H. Graziano Columbia University, New York, NY, USA F. Jasmine, M.G. Kibriya, H. Ahsan The University of Chicago, Chicago, IL, USA T. Islam, A. Ahmed, M. Rakibuz-Zaman, S. Roy, R. Paul-Brutus, T. Islam U-Chicago Research Bangladesh, Ltd., Dhaka, Bangladesh ABSTRACT: Arsenic exposure from drinking water has been linked to subclinical and clinical outcomes of cardiovascular disease (CVD). However, no large-scale studies have evaluated whether the cardiovascular effects of arsenic exposure could be modified by genetic factors. Using data from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh, we evaluated whether the association between arsenic exposure and CVD risk or carotid artery intima-media thickness (cIMT) differs by single-nucleotide polymorphisms (SNPs) in 18 genes related to arsenic metabolism, oxidative stress, and inflammation and endothelial dysfunction. We found significant interactions between well-water As and two SNPs in ICAM1 and VCAM1 in CVD risk, and of both well-water As and urinary As with 3 SNPs in AS3MT in cIMT. Our data provide novel evidence that the cardiovascular effects of arsenic exposure may vary with some common genetic variants in genes related to arsenic metabolism and endothelial dysfunction. 1 INTRODUCTION Arsenic (As) is a naturally occurring element primarily encountered in drinking water and foods, exposing millions of people worldwide to this toxic agent. Chronic exposure to As from drinking water has been linked to subclinical and clinical outcomes of cardiovascular disease (CVD) (Moon et al. 2012). Carotid artery intima-media thickness (cIMT) is a widely accepted indicator of subclinical atherosclerosis and a valid surrogate marker for clinical endpoints. The litera- ture suggests that As-related health effects may be modified by genetic factors. However, no large-scale studies have evaluated whether the cardiovascular effects of As exposure could be modified by genetic factors. 2 METHODS 2.1 Gene-environment interactions in CVD risk Using the resources from the Health Effects of As Longitudinal Study (HEALS) in Bangladesh, a prospective cohort of more than 20,000 participants recruited in 2000 (Ahsan et al. 2006), we conducted a case-cohort study of 447 incident fatal and nonfatal cases of CVD, including 165 stroke cases and 238 cases of coronary heart disease (CHD), and a subcohort of 1,375 subjects randomly selected from the HEALS to evaluate whether the association between As exposure and risk of CVD, CHD, and stroke dif- fers by single-nucleotide polymorphisms (SNPs) in 18 genes related to As metabolism (GSTM1, GSTT1, GSTO1, GSTP1, MTHFR, CBS, PNP, and AS3MT), oxidative stress (HMOX1, NOS3, SOD2, and CYBA), and inflammation and endothelial dysfunction (APOE, TNF, IL6, ICAM1, S1PR1, and VCAM1). Candidate genes were selected if they 1) are involved in As metabolism, or 2) have been related to As exposure in animal, in vitro, or epidemiologic studies and are known to play a key role in CVD risk in epidemiologic studies. We used a comprehensive approach to select SNPs in the candidate genes of interest. We first selected tag SNPs from International Hapmap Project and SeattleSNPs using the r2-based Tagger program with a pairwise r2 ≥ 0.80 and a minor allele frequency (MAF) ≥ 5%. The selection was performed for each ethnic group in the Hapmap/SeattleSNPs data separately to compile a list that includes all the tag SNPs. We also selected validated, non-synonymous SNPs with a MAF ≥ 5% from SeattleSNPs and potentially functional SNPs from the F-SNP database (Lee and Shatkay 2008). In addition, we included SNPs that have been related to CVD risk and/or phenotypic markers of interest in the literature. After removing SNPs with genotyping efficiency < 95%, monomorphic genotype data, Hardy-Weinberg equilibrium < 0.0001, and an MAF < 5% in the study population, a total of 170 SNPs in 17 genes were remained for analysis. We assessed the multiplicative interaction by the cross-product term of As exposure and each candidate SNP using the Cox proportional hazards models. We also assessed interaction on the additive scale (synergy) by testing whether the joint effect of As exposure and a SNP was greater than the sum of their independent effects. 2.2 Gene-environment interactions in cIMT We conducted a cross-sectional study of 1078 participants in the subcohort to evaluate gene-environment interactions between As exposure and the abovementioned SNPs in cIMT. We used the mean of the near and far walls of the maximum common carotid artery IMT from both sides of the neck (mean of 4 sites) as the main outcome variable. The multiplicative interaction between As exposure and each SNP was assessed using multiple linear regression models. cIMT (β) in relation to every standard deviation (SD) increase in As exposure alone, presence of each SNP alone, and the joint effect of As exposure and each SNP was assessed. 3 RESULTS 3.2 Interactions between As exposure and SNPs in cIMT Although not significant after correcting for multiple testing, nine SNPs in APOE, AS3MT, PNP, and TNF genes had a nominally significant interaction with well-water As in cIMT. cIMT in relation to the joint effect of both higher well-water As exposure and CT or TT genotype of APOE rs7256173 (β = 46.0 µm, 95% CI = 10.7, 81.3), CC genotype of AS3MT rs10883790 (β = 35.8 µm, 95% CI = 9.2, 62.4), AA genotype of rs11191442 (β = 38.3 µm, 95% CI = 12.1, 64.5), and GG genotype of rs3740392 (β = 40.9 µm, 95% CI = 14.4, 67.5), was greater than the cIMT in relation to the genotype alone (β = -8.1 µm, -5.4 µm, -5.8 µm, and -5.1 µm, respectively) or As exposure alone (β = 8.7 µm, 8.0 µm, 7.8 µm, and 7.2 µm, respectively). These SNPs also showed similar pattern of interactions with urinary As. Additionally, the at-risk genotypes of the AS3MT SNPs were positively related to increased proportion of monomethylarsonic acid (MMA) and negatively related to proportion of dimethylarsinic acid (DMA) in urine, indicators of suboptimal As methylation capacity. 3.1 Interactions between As exposure and SNPs in risk of CVD, CHD, and stroke 4 CONCLUSIONS The multiplicative interactions between well-water As and two SNPs, rs281432 in ICAM1 (Padj = 0.0002) and rs3176867 in VCAM1 (Padj = 0.035) in CVD risk, were significant after adjustment for multiple testing of all 170 tests. The hazard ration (HR) for CVD risk was 1.82 [95% confidence interval (CI): 1.31, 2.54] for every SD increase (101.3 µg/L) in well-water As among those with GG genotype of rs281432, much greater than the HR of 0.96 (95% CI: 0.65, 1.42) associated with GG genotype alone or the HR of 1.08 (95% CI: 0.94, 1.25) associated with one SD increase in well-water As alone. The magnitude of the interaction between well-water As and rs3176867 in VCAM1 was weaker; the HR for CVD risk was 1.34 (95% CI: 0.95, 1.87) for one SD increase in well-water As among those with CC genotype of rs3176867. These associations were similar for stroke risk but weaker for CHD risk. Our data provide novel evidence that genetic variants in genes related to endothelial dysfunction may modify the risk of CVD associated with As exposure whereas genetic variants in genes involved in As metabolism may play a more important role in Asinduced subclinical atherosclerosis. For main effects of SNPs, AA genotype of NOS3 rs2853792 and GG genotype of SOD2 rs5746088 were associated with a significantly reduced risk of CVD (HR = 0.51, 95% CI: 0.38, 0.69; and 0.30, 95% CI: 0.17, 0.51, respectively) and CHD (HR = 0.49, 95% CI: 0.34, 0.70; and 0.29, 95% CI: 0.15, 0.58, respectively). CC genotype of MTHFR rs1801133 was related to a significantly increased risk of stroke (HR = 2.33; 95% CI: 1.51, 3.61). ACKNOWLEDGEMENTS This work is supported by grants R01ES017541, P42ES010349, P30ES000260, and R01CA107431 from the National Institutes of Health. REFERENCES Moon, K., Guallar, E., Navas-Acien, A. 2012. Arsenic exposure and cardiovascular disease: an updated systematic review. Current atherosclerosis reports 14(6): 542-555. Ahsan, H., Chen, Y., Parvez, F., Zablotska, L., Argos, M., Hussain, I., et al. 2006. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the Health Effects of Arsenic Longitudinal Study. Am J Epidemiol 163(12): 1138-1148. Lee PH, Shatkay H. 2008. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic acids research 36: D820-824.