Lab # 11 Report
advertisement

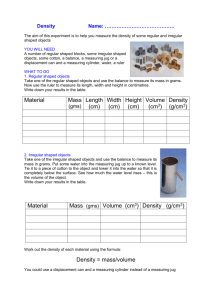
PHY122 Lab # 11 NAME __________________ DENSITY Lab Partners: Purpose: The purpose of this lab is to gain practice using various types of devices and techniques for making measurements, and studying the property called "density". Apparatus 2 rectangular shaped solids made of different materials 2 metal cylinders made of different materials 1 irregularly shaped object 1 triple beam balance 1 overflow tank with capture bucket 1 vernier caliper 1 piece of string 1 spring scale Procedure Part A 1) Using the vernier calipers measure and record the length, width, and height of the first rectangular shaped object. 2) Calculate and record the volume of the first rectangular shaped object. 3) Using the triple beam balance, measure and record the mass of the first rectangular shaped object. 4) Calculate and record the mass density of the first rectangular shaped object in grams/cubic centimetres. 5) Repeat steps 1 to 4 using the second rectangular shaped object. 6) Using the vernier calipers measure and record the diameter and height of the first metal cylinder. 7) Calculate and record the volume of the first metal cylinder. 8) Using the triple beam balance, measure and record the mass of the first cylinder. 9) Calculate and record the mass density of the first cylinder in grams/cubic centimetres. 10) Repeat steps 6 to 9 using the second cylinder. Data and Results for Part A Rectangular Object # 1 Object Rectangular Object # 2 Cylindrical Object # 1 Cylindrical Object # 2 Length Width Height Diameter Volume Mass Mass Density Procedure Part B 11) Place the overflow tank so that the overflow tube is over the sink. 12) Fill the overflow tank with water until it comes out of the overflow tube. 13) Measure and record the mass of the irregularly shaped object. 14) Measure and record the mass of the capture bucket. 15) Place the capture bucket under the overflow tube so that it will catch any water that comes out of the tube. 16) Lower the irregularly shaped object into the overflow cylinder so that it is completely submerged. 17) Measure and record the mass of the capture bucket with the water inside it. 18) Calculate and record the mass of the water that was displaced. 19) Calculate the volume of the water that was displaced based on mass of water. 20) Calculate the mass density of the irregularly shaped metal object in g/cm3. Mass of the irregularly shaped object Mass of empty capture bucket _______________ _______________ Mass of capture bucket with water _______________ Mass of water alone _______________ Volume of water _______________ Mass Density of the irregularly shaped object _______________ Note: any time that an object is submerged inside a fluid, that object will weigh less by an amount equal to the weight of the fluid displaced. Procedure Part C Archimedes’ Principle 21) Take the irregularly shaped objects and tie it to the spring scale. 22) Measure its mass when suspended in air. 23) Measure its mass when fully submerged. 24) Determine the difference in the readings. (This is the mass of the water displaced.) 25) Calculate the specific gravity of the irregularly shaped object. Step in procedure 22) Measured quantity 23) 24) Mass measured under water Mass of water displaced 25) Specific gravity of object Mass as measured in air Measured amount Questions for the Density Lab NOTE: Base your answers to the questions below on the data tables given in the PHY122 Lab # 11 Introduction file. 1) What are the units for specific gravity? Why? 2) What is the weight density of carbon dioxide gas in lb/cubic foot? (Assume 0 degrees Celsius and 1 atm as per the values listed in the tables.) 3) What is the relative density of ice? 4) Why is the specific gravity of carbon dioxide larger than the specific gravity of ice? Does this mean that pure carbon dioxide is denser than ice?