Free Energy, Vapor Pressure and the Equilibrium Between a Vapor
advertisement

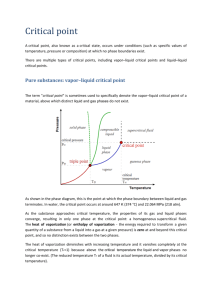
Free Energy, Vapor Pressure and the Equilibrium Between a Vapor and Condensed Phase (Entirely plagiarized from Physics 5719, “The Physics of Nuts and Bolts”, aka “Lab Survival Skills”) References: Thermodynamics, G. N. Lewis and M. Randall, rev. K. Pitzer and L. Brewer, McGraw Hill, New York (1961). Chemical Thermodynamics, I. Klotz, Benjamin, New York (1964). The Gibbs Free Energy is generally agreed to be the “weapon of choice” for describing (a) chemical reactions and (b) equilibria between phases. It is defined as: G = H – TS = E + PV – TS (1) Where H = Enthalpy E = Total internal energy T = [Absolute] Temperature S = Entropy Obviously dG = dE + PdV +VdP – TdS – SdT But from the first Law of Thermodynamics dE = TdS – PdV Hence: since dS = δQ/T and the mechanical work done on a system when it expands is –PdV. dG = -SdT + VdP Let us apply this to a closed system containing a pure substance consisting of its vapor and a condensed phase co-existing in equilibrium. The objective will be to see how the pressure of the vapor depends on the temperature of the system. Clearly we may write the above equation twice, once for each phase: dGc = -ScdT + VcdP where c refers to the condensed phase dGv = -SvdT + VvdP where v refers to the vapor phase The objective of the exercise is to change the temperature of the system (obviously the vapor and condensed phase are in thermal equilibrium, at the same temperature) and see how the pressure of the vapor changes. The system remains in thermal equilibrium if the molar free energy of the system, also known as the chemical potential, remains constant. The definition of chemical equilibrium between two phases is that the free energy is the same in both phases: Gc = Gv,. Hence: dGc = dGv Changes in free energy when some independent variable is changed must be the same if they are to remain in equilibrium. -ScdT + VcdP = -SvdT + VvdP (Sv - Sc )dT = (Vv- Vc)dP dP/dT = (Sv – Sc)/(Vv – Vc) = ΔHv/(TΔV) Here we are talking about the entropy change associated with the reversible, isothermal evaporation of a small quantity of material from a condensed phase to the vapor phase. The entropy change in such a process is simply the heat of vaporization, ΔHv, divided by the temperature at which the vaporization took place, the transition temperature. This is the Clapeyron equation (E. Clapeyron, J. ẻcole polytech. (Paris) 14(23), 153 (1834). It relates the change in pressure of a vapor to the temperature in a closed, mono-component system to the heat of vaporization, system temperature and molar volume change of the material on vaporization. For lack of a better model, we treat most vapors as ideal gases, whose molar volume is given by: V/n = RT/P Under most circumstances, the molar volume of the vapor is about three orders of magnitude larger than the molar volume of the condensed phase. Hence the required volume change can be approximated as the volume of the vapor alone, which is a constant for the isothermal transfer of mass at equilibrium we are discussing and is given by the above expression when calculating equations of state. dP = (ΔHv/Vv)dT/T = (PΔHv/RT)dT/T dP/P = ΔHv/R)dT/T2 ln(P/ P0) = -(ΔHv/R)(1/T – 1/T0) P = P0 exp(-ΔHv/R(1/T – 1/T0)) The vapor pressure in equilibrium with a condensed phase increases exponentially (sort of: exp(-1/T) isn’t exactly an exponential!) with temperature from zero up to the critical temperature. This is borne out in the vapor pressure charts and generating functions for them. Deviations from linearity on the log-log plot reflect the temperature dependence of the heat of vaporization and the fact that exp (-1/T) isn’t really linear in the exponent. Vapor pressure of some elements; stolen from VEECO website. Vapor pressure data are given in the CRC Handbook in the form Log10p(Torr) = -0.2185*A/T + B For water, CRC gives: A = 10999.4, B = 9.183837 Plotting this gives: Vapor Pressure of Water Vapor Pressure (Torr) 10000 "Normal boiling point" 1000 100 10 1 0.1 -20 0 20 40 60 80 100 Temperature (C) The first thing we see is that the generating function is imperfect: the normal boiling point of water on the plot is about 108.2 C. (I cheated; I looked at the data!) 120 Kubaschewski, Evans and Alcock, Metallurgical Thermochemistry, Pergammon, Oxford (1967), use the higher order expression: Log10p(Torr) = A/T + BlogT + CT + D Vapor Pressure of Water Vapor Pressure (Torr) 10000 "Normal boiling point" 1000 100 10 Kubaschewski et al. 1 0.1 -20 0 20 40 60 80 100 120 Temperature (C) In this case the vapor pressure at 100 C is 758 Torr, a much better approximation! Caveat emptor! Great resource tool: the “RCA Charts”, which live in 327 JFB. Treat them with great respect: they are much older than you are and irreplaceable. Phase diagram of water. So what does all this mean? Vapor pressure is a continuous function of temperature for all temperatures from absolute zero to the critical point, above which only a single phase is defined. The melting point on a vapor pressure curve conveys no significance whatsoever. The vapor pressure of water at 0 C is 4.6 Torr; it’s just another point on an otherwise smooth curve. Actually this isn’t quite true: because the solid and liquid have different heat capacities, the vapor pressure curve changes slope at the melting point. The vapor pressure still changes continuously across the melting point and is of no particular significance there. Homework: Find or calculate the vapor pressures of CO2 and gallium at their melting points. The Clapeyron Equation can be used equally well to determine the decrease in the freezing point of water with increasing pressure. This is an interesting calculation that apparently gives the wrong answer to the question: “what makes ice skates work?”. Note that this is not the normal case: the freezing point decreases with increasing pressure only because water expands on freezing; the solid is less dense than the liquid! (You know this: ice floats on water.) Conversely, the freezing point of water increases with decreasing pressure. Hence the Triple Point, at which all three phases, solid, liquid and vapor, coexist is 0.01 C. (Actually this is now a “defined temperature”.) “Normal boiling point”. At 95 C, the generating function says the vapor pressure of water is 633 Torr; at 97 C the generating function says 657 Torr. Ambient pressure in SLC is about 640 Torr, obviously depending on the weather. A pan on a stove is heated from below. Convection in the water distributes heat and establishes a reasonable facsimile of thermal equilibrium. At 95 C a vapor nucleus in the liquid, having an internal pressure of only 633 Torr, will be compressed by the surrounding isostatic pressure; at 97 C a vapor nucleus will have an internal pressure greater than the ambient isostatic pressure and will expand and rise due to a net buoyant force. This is called “boiling”. Attempting to raise the temperature further is futile: any heat which flows into the system, which is at a constant pressure of 640 Torr, is consumed by the latent heat of vaporization and simply converts more material to the vapor phase. Because raising the temperature above the boiling point would create an imbalance in mechanical equilibrium between the internally generated vapor and the externally imposed ambient pressure, it is impossible to raise the temperature above the boiling point as long as there is condensed phase in the system. This statement is independent of ambient pressure; conversely, the temperature at which this “boiling” phenomenon takes place depends strongly on pressure. Homework: what is the boiling point of water in a vacuum system at 10-6 Torr? So what does all this have to do with an ESEM???? As you know, the NovaNano is a variable pressure machine that bleeds in water to passivate the accumulated charge. The Quanta has a thermoelectric cold stage that reduces sample temperatures to 0 +/- 1- C. The Quanta bleeds in even higher pressures of water than the NovaNano so that the sample is in thermodynamic equilibrium with its environment at temperatures of 0 +/- 10 C!