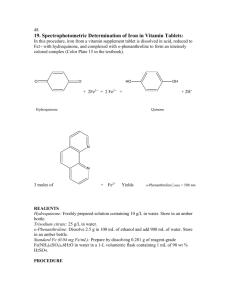
HYDROQUINONE 0.0 OVERVIEW A. Hydroquinone is a
advertisement

HYDROQUINONE 0.0 OVERVIEW A. Hydroquinone is a depigmenting agent. B. DOSING INFORMATION: The usual dose of topical hydroquinone 2% to 4% is one application twice daily. C. PHARMACOKINETICS: The onset and duration of depigmentation produced by hydroquinone varies among individuals. D. CAUTIONS: The application of hydroquinone should be limited to relatively small areas of the body at any one time. This agent should not be used in the eyes or on open wounds, and if skin irritation develops, hydroquinone should be discontinued immediately. E. CLINICAL APPLICATIONS: Hydroquinone is used for reversible depigmentation of hyperpigmented skin due to conditions such as freckles, senile lentigines, melasma, and chloasma. 1.0 DOSING INFORMATION 1.1 DOSAGE FORMS A. Information on specific products and dosage forms can be obtained by referring to the Product Index. 1.3 ADULT DOSAGE 1.3.1 NORMAL DOSE A. TOPICAL 1. The usual dose of hydroquinone 4% topical preparations in the treatment of HYPERPIGMENTATION is one application twice daily to the affected area(s). If no response is noted after 2 months of therapy, hydroquinone should be discontinued (Prod Info Solaquin Forte(R), 1994; Prod Info Eldopaque Forte(R), 1994; Prod Info Eldoquin Forte(R), 1994). 2.0 PHARMACOKINETICS 2.3 ADME 2.3.1 ABSORPTION A. TOPICAL, 35% (Bucks et al, 1988). 3.0 CAUTIONS 3.2 PRECAUTIONS A. Limit to relatively small areas of the body at any one time. B. Do not use in eyes or on open wounds. C. If irritation develops, discontinue immediately. 3.3 ADVERSE REACTIONS 3.3.6 KIDNEY/GENITOURINARY A. URINE DISCOLORATION See Drug Consult reference: "URINE DISCOLORATION - DRUG AND DISEASE INDUCED" 3.3.8 OCULAR A. OCULAR EFFECTS 1. CONJUNCTIVAL CHANGES have been associated with hydroquinone exposure (Reynolds, 1990). 3.3.10 SKIN A. DERMATOLOGIC EFFECTS 1. In a randomized, double-blind, comparative study of azelaic acid 20% and hydroquinone 2% cream for the treatment of melasma in 155 patients, side effects from both preparations consisted of transient mild to moderate irritant reactions. Symptoms of BURNING, ITCHING and/or STINGING occurred in 9 of 78 patients receiving hydroquinone (Verallo-Rowell et al, 1989). 2. Transient ERYTHEMA, mild burning, stinging, DRYNESS and fissuring of paranasal and infraobital areas have been reported with hydroquinone use (Reynolds, 1990; Fisher, 1983). B. VITILIGO 1. Isolated cases of widespread contact LEUKODERMA and contact vitiligo have been reported with topical hydroquinone use (Fisher, 1983; Boyle & Kennedy, 1985; Romaguera & Grimalt, 1985; George, 1989). 2. Confetti-like DEPIGMENTATION occurred in a 63-year-old male diabetic following the daily application to the beard area of a hydroquinone-containing cream for a 3-month period (Ambi Extra for Men(R)) (Markey et al, 1989). Gradual repigmentation of the affected area began 4 months after discontinuation of the Ambi cream. C. DERMATITIS 1. Localized CONTACT DERMATITIS has been reported with topical hydroquinone use (Prod Info Solaquin Forte(R), 1994). 2. Contact dermatitis developed in a 34-year-old female following the use a cream containing hydroquinone (strength not reported) and PABA (para-aminobenzoic acid) for treatment of chloasma. Patch test revealed a positive reaction to hydroquinone 1% (Romaguera & Grimalt, 1985). D. OCHRONOSIS 1. SUMMARY: Ochronosis secondary to the application of hydroquinone-containing creams has been reported by several investigators. This paradoxical hyperpigmentation of the skin is usually associated with long-term use of hydroquinone bleaching creams. The hyperpigmentation usually fades following discontinuation of the cream; however, in severe cases, it may be irreversible (Anon, 1987; Brauer, 1985; Weiss et al, 1990). 2. Ochronosis-like PIGMENTATION described as pitch-black pigmentation and colloid millium formation was noted following the use of 2% hydroquinone-containing creams in 2 black American patients. These skin changes were accentuated in areas of sun exposure (Hoshaw et al, 1985). 3. Exogenous ochronosis occurred in a 36-year-old MexicanAmerican female following the application of hydroquinone 2% cream for less than 6 months (Howard & Furner, 1990). 4. SARCOID-LIKE OCHRONOSIS developed following treatment with hydroquinone 4% cream for dark spots (possibly a form of melasma). A discrete, nonpruritic papular eruption was noted on both cheeks of the patient, which was successfully treated with tetracycline with resolution of the lesions in 3 months (Fisher, 1988). E. NAIL DISCOLORATION 1. FINGERNAIL STAINING due to hydroquinone-containing cream was reported in 2 older females (Mann & Harman, 1983). Both patients had applied hydroquinone 2%-containing cream twice daily to the back of their hands for actinic lentigines. The staining was described as a brown discoloration. F. HAIR CHANGES See Drug Consult reference: "DRUG-INDUCED HAIR CHANGES" 3.3.12 OTHER A. OVERDOSE See POISINDEX(R) Management "HYDROQUINONEQUINONE" B. HYPERSENSITIVITY 1. Occasional hypersensitivity reactions have occurred following hydroquinone use, and skin sensitivity testing is recommended by applying a small amount of hydroquinone on an unbroken patch of skin for 24 hours (Prod Info Solaquin Forte(R), 1994; Fisher, 1983; Ketel, 1984). 3.4 TERATOGENICITY/EFFECTS IN PREGNANCY A. TERATOGENICITY 1. U.S. Food and Drug Administration's Pregnancy Category C (Prod Info Eldoquin Forte(R), 1997). See Drug Consult reference: "PREGNANCY RISK CATEGORIES" 4.0 CLINICAL APPLICATIONS 4.2 PATIENT INSTRUCTIONS HYDROQUINONE (HYE-droe-kwin-one) (FOR THE SKIN): - Lightens freckles and brown patches on the skin (liver spots). BRAND NAME(S): Eldopaque Forte(R), Eldoquin Forte(R), Solaquin Forte(R), Melanex(R) WHEN YOU SHOULD NOT TAKE THIS MEDICINE: - Do not use this medicine if you have had an allergic reaction to hydroquinone. HOW TO TAKE AND STORE THIS MEDICINE CREAM/LOTION/GEL/SOLUTION: - Your doctor will tell you how much to use and how often. - Use only on the skin. - Put a small amount of medicine on the skin spots. - Keep the medicine away from your eyes and mouth. - If you do get medicine in your eyes, rinse your eyes with water and call your doctor. - Wash your hands with soap and water after using this medicine. - Store the medicine at room temperature away from heat and moisture. Do not freeze. - Keep all medicine out of the reach of children. IF YOU MISS A DOSE: - Use the missed dose as soon as possible. - If it is almost time for your next regular dose, wait until then to use the medicine and skip the missed dose. - Do not put on two doses at the same time. DRUGS AND FOODS TO AVOID: Ask your doctor or pharmacist before taking any other medicine, including over-the counter products. WARNINGS: - If you are pregnant or breastfeeding, talk to your doctor before using this medicine. - Make sure your doctor knows if you have other medical problems. - Protect your skin from the sun with clothing or sunscreen while using this medicine. SIDE EFFECTS Call your doctor right away if you have any of these side effects: - Skin rash, redness, swelling, or intense itching If you have problems with these less serious side effects, talk with your doctor: - Itching or mild stinging IF YOU HAVE OTHER SIDE EFFECTS THAT YOU THINK ARE CAUSED BY THIS MEDICINE, TELL YOUR DOCTOR 4.3 PLACE IN THERAPY A. Hydroquinone can be used to lighten hyperpigmented areas of the skin associated with chloasma, melasma, freckles, senile lentigines, and other forms of hyperpigmentation caused by melanin. B. Theoretically, hydroquinone has the potential to be an effective agent for the treatment of hypermelanosis. Additionally, several clinical studies comfirm its efficacy as a bleaching agent. However, adverse effects (ie, unpredictable skin sensitization in some patients, reversal of depigmentary effects from subsequent exposure to ultraviolet light) and incomplete disappearance of pathologic hypermelanosis following treatment may restrict its use. 4.4 MECHANISM OF ACTION/PHARMACOLOGY A. MECHANISM OF ACTION 1. Hydroquinone produces reversible depigmentation of the skin by suppression of melanocyte metabolic processes, in particular the inhibition of the enzymatic oxidation of tyrosine to DOPA (3,4-dihydroxyphenylalanine) (Prod Info Solaquin Forte(R), 1994). Sun exposure reverses this effect, and will cause repigmentation (Reynolds, 1990). B. REVIEW ARTICLES 1. Efficacy of hydroquinone as a bleaching cream is discussed (Hart & Yi, 1993). 4.5 THERAPEUTIC USES A. HYPERPIGMENTATION FDA Labeled Indication 1. OVERVIEW: FDA APPROVAL: Adult, yes; pediatric, yes (over 12 years) EFFICACY: Adult, effective; pediatric, effective DOCUMENTATION: Adult, good; pediatric, good 2. SUMMARY: - Used to lighten hyperpigmented areas of the skin associated with CHLOASMA, MELASMA, FRECKLES, SENILE LENTIGINES, and other forms of hyperpigmentation caused by melanin (Prod Info Solaquin Forte(R), 1994; Fisher, 1983) 3. ADULT: a. Addition of kojic acid to hydroquinone and glycolic acid combination may improve efficacy in the treatment of MELASMA. Forty Chinese women received twice a day 2% hydroquinone and 10% glycolic acid combination gel in one half of the face, and received 2% kojic acid, 2% hydroquinone, and 10% glycolic acid combination gel on the other half of the face in a double-blind randomized manner. After 12 weeks, patients with greater than 50% improvement in melasma were found in 60% of the side with kojic acid and 48% of the side without kojic acid. Both formula improved melasma, but the difference between the 2 was not statistically significant (p=0.9). Three patients developed redness and irritation and withdrew from the study. All patients experienced redness, stinging, and mild exfoliation on both sides of the face. The adverse effects were settled by the third week. Kojic acid did not worsen side effects and could be added to the treatment regimen if patients did not respond to the hydroquinone and glycolic acid combination (Lim, 1999). 4. PEDIATRIC: a. Resolution of hyperpigmentation secondary to a JELLYFISH STING was described in a 17-year-old patient following treatment with topical hydroquinone 1.8% twice daily for 40 days (Kokelj & Burnett, 1990). 4.6 COMPARATIVE EFFICACY AND EVALUATION WITH OTHER SIMILAR THERAPEUTIC AGENTS A. AZELAIC ACID 1. MELASMA: a. Relatively large double-blind studies have reported that topical azelaic acid 20% cream is superior to topical hydroquinone 2% cream (Verallo-Rowell et al, 1989) and comparable in efficacy to hydroquinone 4% cream (Fitton & Goa, 1991; Balina & Graupe, 1991) in the treatment of melasma. The incidence of local adverse effects with 20% azelaic acid cream and 2% to 4% hydroquinone were comparable (Fitton & Goa, 1991).