Burana Ibuprofen: Package Leaflet Information
advertisement

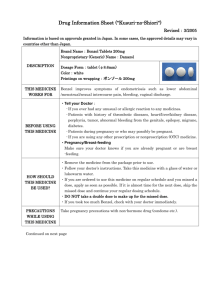
PACKAGE LEAFLET: INFORMATION FOR THE USER Burana 200 mg film-coated tablet (tablet) Burana 400 mg film-coated tablet (tablet) Burana 600 mg film-coated tablet (tablet) Ibuprofen Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it again. If you have any further questions, ask your doctor or pharmacist. This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours. If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. In this leaflet: 1. What Burana is and what it is used for 2. Before you use Burana 3. How to use Burana 4. Possible side effects 5. How to store Burana 6. Further information 1. WHAT BURANA IS AND WHAT IT IS USED FOR Burana belongs to a group of medicinal products named NSAID (non-steroidal anti-inflammatory drugs). Burana reduces fever and has a pain-relieving and anti-inflammatory effect. The effect is achieved usually in 30 minutes and the maximal effect in 1-2 hours. Burana 200 mg and 400 mg tablets are used for acute painful conditions of mild to moderate intensity like headache, toothache, back pain, muscle- and joint pain, fever associated with upper respiratory tract infection (e.g. common cold) and menstrual pains. Burana 400 mg and 600 mg are used for symptomatic treatment of pain and inflammation in rheumatic conditions (rheumatoid arthritis and osteoarthritis) in adults. 2. BEFORE YOU USE BURANA Do not use Burana if you are allergic (hypersensitive) to ibuprofen or any of the other ingredients of Burana if you have experienced allergic reactions like asthma, rhinitis, or urticaria with intake of painrelieving drugs containing acetylsalicylic acid or certain other anti-inflammatory drugs (NSAIDs) if you have increased risk for bleeding if you have gastric or duodenal ulcer or have had recurrent gastric or duodenal ulcer (two or more distinct episodes of proven ulceration or bleeding) if you have experienced gastrointestinal bleeding or ulceration related to treatment with NSAIDs if you have severe hepatic failure if you have severe heart failure or severe impaired renal function if you are in the last three months of pregnancy. Take special care with Burana if you have kidney or liver disease if you have heart failure if you have hypertension if you have asthma or an allergic disease 1 - if you have SLE (Systemic lupus erythematosus) a collagen disease if you have had gastric or duodenal ulcer or other gastrointestinal conditions (like ulcerative colitis, Crohn’s disease) if you have a disease associated with increased tendency for bleeding if you are suffering from significant dehydration (caused by vomiting, diarrhoea or insufficient fluid intake). Consult your doctor before taking Burana if you are pregnant or are planning a pregnancy. See also section “Pregnancy, breast-feeding and fertility”. Elderly people should be aware of their increased risk of adverse events, especially bleeding and perforation in the digestive tract, which may be fatal. If you have earlier had an ulcer in the stomach or intestines, especially if this has been complicated by perforation or accompanied by bleeding, you should look out for any unusual symptoms in the abdomen, and report them at once to your doctor. Bleeding, ulceration or perforation in the stomach or intestines may occur without any warning signs even in patients who have never had such problems before. If bleeding or ulceration of the digestive tract occurs, the treatment has to be stopped. In patients with systemic lupus erythematosus (SLE) and mixed connective tissue disorders there may be an increased risk of aseptic meningitis. The lowest effective dose should always be used to minimize the risk for adverse events. Using a higher dose than recommended may increase the risk for adverse events. Stop taking Burana and contact immediately your doctor if you experience swelling of among others face, tongue, and/or throat or nettle-rash, shortness in breath and difficulty in swallowing (angiooedema). Serious skin reactions have been reported very rarely in association with the use of NSAIDs. Stop taking Burana and contact a doctor if you get skin reactions or mucosal lesions. During varicella it is advisable to avoid use of this drug. Medicinal products like Burana may involve a small increased risk of myocardial infarction or stroke. This small increased risk is more likely at high dosages and in long term treatment. Ask your doctor or pharmacist for advice about your treatment if you have any heart disease, if you earlier have had stroke or if you believe you have an increased risk for these conditions (e.g. if you have hypertension, diabetes, high cholesterole values or if you are smoking). Using other medicines Do not use simultaneously other pain-killers, including other NSAIDs, without first consulting a doctor or pharmacist. Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. The effect of the following medicines may change or the effect of Burana may change in concomitant use: acetylsalicylic acid (drugs against pain and fever, and in small doses to prevent blood clots) warfarin, ticlopidin (certain drugs for blood clots) methotrexate (drugs for cancer and immune system disturbances) antihypertensive drugs like beta blockers, diuretics (thiazides, furosemide, bumetanide), ACE inhibitors (e.g. captopril) and angiotensin II-antagonists (e.g. losartan) cyclosporin, tacrolimus (drugs that prevent transplant rejection) lithium (drugs for manic-depressive illness) selective serotonin reuptake inhibitors (so called SSRIs, e.g. paroxetine, sertraline, citalopram against depression) corticosteroids (anti-inflammatory drugs) 2 - aminoglycosides (certain antibiotics) voriconazole and fluconazole (drugs that treat fungal infections) colestyramine (cholesterol medicine) digoxin (heart medicine). Consult a doctor before using Burana if you use any above mentioned medicine. Pregnancy, breast-feeding and fertility Pregnancy Pregnant women should not use Burana in the last three months of pregnancy. Intake of Burana should be avoided by women who plan pregnancy or are pregnant. Treatment during any part of pregnancy should take place only by doctor’s order. Breast-feeding Ibuprofen is excreted in breast milk but is not likely to have an effect on the infant. Consult a doctor if you use Burana more often than temporarily while breastfeeding. Fertility Use of Burana may temporarily make it more difficult to become pregnant and therefore treatment is not recommended to women who try to get pregnant. This effect is reversible when you stop taking the medicine. Driving and using machines Treatment with Burana may cause visual disturbances as an adverse effect. These effects are uncommon, but must be kept in mind when sharp attention is needed, e.g. when driving a car. Important information about some of the ingredients of Burana Burana 200 mg, 400 mg and 600 mg tablets contain sucrose. Burana 600 mg tablets contain lactose monohydrate. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product. 3. HOW TO USE BURANA Your doctor will adjust the correct dose individually for you. Always take Burana exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. Burana tablets 200 mg and 400 mg Mild to moderate acute painful conditions and fever associated with upper respiratory tract infection (e.g. common cold). The usual dose for adults and children over 12 years: 200-400 mg as a single dose or 3-4 times daily (at least 4 hours between doses), however maximal daily dose is 1200 mg. Single doses over 400 mg do not give any better pain-relieving effect. The usual dose for children 6-12 years (over 20 kg): 200 mg 1-3 times daily (at least 6-8 hours between doses). Menstrual pain. The usual dose for adults and children over 12 years: 400 mg 1-3 times daily (at least 4 hours between doses) when needed. Start taking medicine as soon as menstruation begins to have a better effect. Burana tablets 400 mg and 600 mg Rheumatic diseases. The usual dose for adults: 400 mg - 600 mg 3 times daily. Maximal daily dose is 2400 mg. To the faster relief of early morning stiffness the first dose can be taken on an empty stomach. Elderly 3 If you are elderly you should always consult your doctor before using Burana since you will be more prone to side effects, especially bleeding and perforation in the digestive tract, which may be fatal. Your doctor will advise you accordingly. Reduced kidney or liver function If you suffer from reduced kidney or liver function, always consult your doctor before using Burana. Your doctor will advise you accordingly. If you use more Burana than you should If you have taken a too large dose or if a child has taken Burana by mistake, always contact a doctor or hospital for advice. If you have any further questions on the use of this product, ask your doctor or pharmacist. 4. POSSIBLE SIDE EFFECTS Like all medicines, Burana can cause side effects, although not everybody gets them. Common (occurs in more than 1 out of 100 users): Gastrointestinal side-effects (dyspepsia, abdominal pain, diarrhoea, constipation, nausea, vomiting). Tiredness, headache. Eczema. Gastrointestinal problems usually decreases if you take Burana together with food. Uncommon (occurs in less than 1 out of 100 users): Asthma, itching, nettle-rash, purpura, rhinitis, insomnia, mild anxiety. Hearing and vision disturbances, that disappear when treatment is stopped. Swelling of mucous membranes in mouth and throat (angiooedema). Gastric and intestinal hemorrhages, gastric ulcer. Rare (occurs in less than 1 out of 1000 users): Fluid retention (oedema). Change in blood composition (leucopenia, thrombocytopenia, aplastic anaemia). Heart failure, hypertension. Ulcerative colitis, pancreatitis, perforations, black faeces, flatulence, vomiting of blood, impairment of ulcerous colitis, and Crohn’s disease. Aseptic meningitis, dizziness, inflammation of optic nerve. Anaphylactic reactions (hypersensitive reactions with fever, eczema, oedema and hypotension), liver and kidney effects, skin reactions like in Stevens-Johnsons syndrome, epidermolysis (occasionally serious), erythema, sensitivity to light, hair loss. Depression, confusion, poor vision, ringing in the ears. Exceptional serious infections of the skin in case of varicella. Medicinal products like Burana may involve a small increased risk of myocardial infarction or stroke. If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. 5. HOW TO STORE BURANA Keep out of the reach and sight of children. Burana 200 mg and 400 mg tablets: PVC/Al blister and HDPE jar: This medicinal product does not require any special storage conditions. Burana 600 mg tablets: PVC/Al blister: Store below 30C. HDPE jar: This medicinal product does not require any special storage conditions. Do not use Burana after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month. 4 Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment. 6. FURTHER INFORMATION What Burana contains - The active substance is ibuprofen. The tablets contain 200 mg, 400 mg, or 600 mg ibuprofen, respectively. The other ingredients in the tablet core in 200 mg and 400 mg tablets are colloidal anhydrous silica, microcristalline cellulose, pregelatinised starch, dextrate, croscarmellose sodium and magnesium stearate. The other ingredients in the tablet core in 600 mg tablets are lactose monohydrate, gelatin, macrogol, colloidal anhydrous silica, croscarmellose sodium and microcristalline cellulose. The other ingredients in the film coating are sucrose, hypromellose, polysorbate 80, titanium dioxide (E 171), glycerol (85%) and magnesium stearate. What Burana looks like and contents of the pack Film-coated tablet (tablet). Tablet 200 mg: White or almost white, round, convex, film-coated tablet, scored on both sides. Tablet 400 mg: White or almost white, film-coated capsule formed tablet, scored on both sides. Tablet 600 mg: White or almost white, film-coated capsule formed tablet, scored. 200 mg and 400 mg: The tablets can be divided into equal halves, 600 mg: The score line is only to facilitate breaking for ease of swallowing and not to divide into equal doses. Pack sizes: PVC/Al blister: 10, 20, 30 and 50 tablets. HDPE jar with HDPE screw cap: 100 tablets. Not all pack sizes may be marketed. Marketing Authorisation Holder Orion Corporation Orionintie 1 FI-02200 Espoo Finland Manufacturer Orion Corporation Orion Pharma Orionintie 1 FI-02200 Espoo Finland This medicinal product is authorised in the Member States of the EEA under the following names: Czech Republic: Iburion Denmark: Burana Norway: Burana Poland: Iburion Slovakia: Iburion Sweden: Burana 5 This leaflet was last approved in 2011-09-23 6