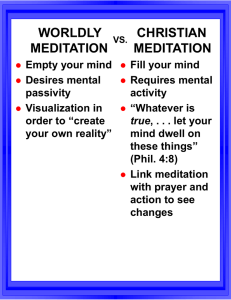
initialreview - Waisman Laboratory for Brain Imaging and Behavior
advertisement

University of Wisconsin-Madison Do not write in this space Application for Initial Review of Research Projects Involving Human Subjects Protocol # Health Sciences IRB ▪ Health Sciences Minimal Risk IRB Training Checked __ Date: I. Application Cover Sheet A. Researcher and Protocol Identification: Impact of long-term training in meditative practices on the hemodynamic brain response to painful stimuli. 1. Protocol Title: 2. Principal Investigator Name: Richard Davidson, Ph. D. Job Title: Professor Department/Unit Name: Department of Psychology Office Address: Waisman Center, Room T225 Phone: 262-8972 Fax: 262-9440 Email: rjdavids@wisc.edu 3. Co-Investigator Name: Antoine Lutz, Ph. D. Job Title: Research Scientist Department/Unit Name: Department of Psychology Office Address: Waisman Center, Room T231 Phone: 262-8705 Fax: 262-9440 Email: alutz@wisc.edu 4. Point of Contact Name: David Perlman Job Title: Graduate Student Department/Unit Name: Department of Psychology Office Address: Waisman Center, Room S109 Phone: 890-1386 Fax: 262-9440 Email: dperlman@wisc.edu -1- Version 1, 2007 July 17 B. Project Sponsorship Information (current or planned): 1. Is the research to be funded with federal funds, or are federal funds being applied for? Yes No 2. Is the research to be funded by a private sponsor? Yes No 3. For each current or potential funding source, provide: a. The name of the sponsoring agency (include UW funding): NIH/NCCAM, U01AT002114-02S1 b. The UW proposal number or planned submission date: 27902 c. The UW grant fund and account number (i.e., 144-abxx): 144-PZ82 A 34 8700 d. The agency award number: NIH/NCCAM, U01AT002114-02S1 C. Additional Project Information: 1. Is this a clinical research project? (Definition: Clinical Research Involving Human Subjects means any research or medical procedure involving human subjects or the use of human samples for the development and evaluation of patient therapies, such as diagnostic tests, drug therapies, or medical devices. It includes clinical trials.) 2. Do any project personnel receive incentives for recruiting human subjects or for any other purpose directly related to the study? If any project personnel receive incentives for recruiting human subjects, this should be described fully in the Study Description section of this application. Yes No Yes No 3. Do any personnel involved in the design, conduct, or analysis of the study have any proprietary interests (royalties, patents, trademarks, copyrights, or licensing agreements) involving any agent, device, or software being evaluated as part of the study? Yes No 4. In addition to the sponsor(s) of this project, are other companies or business entities involved in or potentially affected in a significant way by this research project? If yes, list the names of those companies/business entities. 5. Is this investigator-initiated research? 6. Do you intend to use General Clinical Research Center (GCRC) facilities? Yes No Yes Yes No No -2- Version 1, 2007 July 17 D. Other Approvals: Final approval of the Health Sciences Human Subjects Committee may require review and/or approval by another committee representing the University, its affiliates, a department, or a section. Please submit a notice of review and/or approval by any of the following entities. If review is pending, please indicate the date on which it will occur. Review Required? Yes No Committee University of Wisconsin Comprehensive Cancer Center Clinical Affairs Committee Reviews all cancer-related research protocols. Phone 263-0169 Cardiology Clinical Research Committee Reviews all Cardiology Section research protocols and provides consultation if needed on cardiac issues for protocols originating elsewhere. 263-4856 Yes No Institutional Biosafety Committee / Office of Biological Safety Reviews the research use of recombinant DNA and its derivatives. 263-9026 Yes No Radioactive Drug Research Committee Reviews research involving radiopharmaceuticals that do not deliver an intended clinical benefit or that are not FDA approved. 263-4856 Yes No William S. Middleton Memorial Veterans Hospital (VA) Research and Development Committee Reviews all research protocols involving: 1) UW health sciences researchers with paid appointments at the VA; 2) enrollment of subjects (including use of residual tissue and access to medical records) associated with the VA; or 3) use of VA facilities, e.g. space. 256-1901, ext 7863 or 280-7007 Yes No Research Safety Committee Reviews protocols possessing health hazards, such as gene transfer studies, and protocols intentionally exposing subjects to infectious agents. Meriter Hospital Institutional Review Board 263-8902 Yes No 267-6411 Yes No -3- Review Date Version 1, 2007 July 17 II. Certification of Responsibility for the Application for the Initial Review of Research Involving Human Subjects, Completion of Human Subjects Protection Training Program by Key Personnel, and List of Personnel Who Will Use or Disclose Protected Health Information Please list alphabetically the names, credentials, office addresses, and contact information for ALL University of Wisconsin-Madison investigators and other key personnel who are responsible for the design and conduct of this research. In addition, describe the role of each individual in the conduct of the study, and indicate whether the personnel will use or disclose PHI for this protocol as part of their research duties. Name & Credentials Job Title & Role Contact Information Richard Davidson, Ph.D - Professor - Principal Investigator - Research Scientist - Co-investigator W: (608) 262-8972 rjdavids@wisc.edu W: (608) 262-8705 C: (608) 335-9845 alutz@wisc.edu W: (608) 890-1386 C: (206) 679-0036 dperlman@wisc.edu W: (608) 263-1968 tvsalomons@wisc.edu W: (608) 263-9321 adfrancis@wisc.edu W: (608) 265-9118 jgsheftel@wisc.edu Antoine Lutz, Ph. D. David Perlman - Graduate Student - Contact Tim Salomons Andrew Francis - Graduate Student - fMRI pain task consultant - Staff Jenna Sheftel - Staff Four (+) research assistants - Undergraduate Research Assistants from the Department of Psychology - Research, supervised by investigators Will you request that this person have access to PHI from a primary source (e.g., WISCR, paper charts)? NO Will this person use or disclose PHI? NO YES NO YES NO YES NO YES NO YES NO YES YES As Principal Investigator of this protocol, I take full responsibility for this Application for the Initial Review of Research Involving Human Subjects and certify that the key personnel listed above and I have completed the training module “Human Subjects Protection at the University of Wisconsin–Madison” available at http://www.rsp.wisc.edu/humansubs/training/UWHSTraining.html. I realize that: 1) this certification is to satisfy UW-Madison and NIH policy requirements; and 2) I am accountable for the accuracy of this certification. _____________________________________________ _______________________________________ Principal Investigator’s Signature Date -4- Version 1, 2007 July 17 III. Abstract IN LAY TERMS using 300 words or less, please describe: 1) your research question; 2) your experimental design; 3) the major risks to subjects; 4) the potential benefits to subjects; and 5) your specific consent procedure(s). Please do NOT refer to sections of your protocol or to "see attached" in this abstract. The project’s objective is to study the effect of long-term mental training on pain regulation, and on the brain mechanisms that subserve this effect. Specifically, we hypothesize that compared to controls, longterm practitioners will show increased ability to regulate thermal pain by means of meditation, as measured by self-report; and different neural patterns in response to the thermal pain stimulus and the meditation state itself. To test these hypotheses, the study will recruit 20 long-term meditation practitioners. They will rate the intensity and unpleasantness of thermal pain stimulus administered in the fMRI scanner while alternately practicing meditative and neutral states. Also, 40 age- and gender-matched control subjects inexperienced in meditation will be recruited locally. Control participants will run the thermal pain calibration procedure; those whose pain threshold matches the experimental group will be selected to perform the full task in the scanner. Participants will be paid for participating. To create greater motivation, control participants will have the opportunity to receive a bonus for high performance of the meditation task as measured by fMRI activation. There are no direct benefits to participants. Potential risks associated with fMRI include ferromagnetic collision, neurostimulation effects, and psychological or physical discomfort. Other potential risks include discomfort of painful thermal stimulation, and breach of confidentiality. Consent for the present study will entail both written and verbal descriptions of the protocol. Subjects will be informed that their participation is completely voluntary, and that they can withdraw at any time. -5- Version 1, 2007 July 17 IV. Questionnaire Please answer all of the following questions. If you answer “Yes” to any questions, please include details in Section V, Study Description. “Yes” answers also indicate that additional requirements may apply to your protocol. VULNERABLE GROUPS: 1. Will this study involve minors (people less than 18 years old)? Yes No 2. Will this study involve subjects who have a status relationship (e.g., students or employees) with the principal investigator(s)? Yes No 3. Will this study involve prisoners? If Yes, are control group subjects randomly selected? If No, please provide justification in the study description. Yes Yes No No 4. Will this study recruit psychiatric inpatients or people who are institutionalized (e.g., in a mental health facility, nursing home, or halfway house)? Yes No 5. Will this study include women with childbearing potential? Yes No 6. Will this study exclude fertile women? Yes No 7. Will this study include subjects from the Middleton VA Hospital? Yes No 8. Will this study include adults who have impaired decision-making capacity (e.g., coma, dementia, confusion, or mental disorders)? Yes No 9. Will this study include gametes, embryos, fetuses, or involve tissues from embryos or fetuses? Yes No 10. Will this study target or exclude a particular ethnic or racial group? Yes No Yes No 12. Will this study involve an investigational device? If Yes, please provide the IDE# _____________ and 3 copies of the IDE specifications Yes No 13. Will this study involve the administration to subjects of radiopharmaceuticals that are not FDA approved? If Yes, please contact the secretary for the Radioactive Drug Research Committee (RDRC) at 263-4856. Yes No 14. Will this study store blood or tissue samples beyond publication of the study results? Yes No 15. Will this study use an existing depository or collection of blood or tissue samples? Yes No 16. Will this study do testing for genetic markers on blood or tissue samples? Yes No 17. Will this study involve the administration to subjects of recombinant DNA materials? If Yes, consult the staff of the Biological Safety Office at 263-9026. Yes No SPECIAL PROCEDURES: 11. Will this study involve an investigational new drug (IND)? If Yes, please provide the IND #_______________________ If you hold the IND, please provide 3 copies of the application submitted to the FDA Note: If you answer Yes to question 14, 15, or 16 you should consult HS-IRB Guidelines for Genetic Research and the Use of Storable Tissues for potential consent form language. You should also complete the tissue collection information sheet in those Guidelines and attach it to this form. -6- Version 1, 2007 July 17 SPECIAL POLICIES: 18. Will this study use a placebo? Yes No 19. Will this study potentially reveal that subjects engaged in illegal behaviors or stigmatizing behavior (e.g., illicit drug use, child abuse, alcoholism, or gambling)? Yes No 20. Is this study minimal risk? Yes No 21. Are you requesting a waiver of written consent? Yes No 22. Will this study use advertising, recruitment letters, or recruitment posters to invite subject participation? If Yes, please attach copies of these materials to this form. Yes No 23. Will this study use questionnaires, surveys, or other written assessment instruments? If Yes, please attach of copy of these materials to this form. Yes No 24. Will this study involve non-UW researchers? Yes No 25. Will you conduct this study outside of the United States? Yes No 26. Where will you conduct this study? UWHC [UW-Madison Integrative Medicine] Middleton VA Hospital Meriter Hospital Other site(s) – Please list: Waisman Laboratory for Brain Imaging and Behavior -7- Version 1, 2007 July 17 V. Study Description A. Benefit and Purpose Our goal is to have 16 long-term meditation practitioners and 16 matched control subjects complete the study. The objective of this research project is to gain new insights on the impact of mental training on pain regulation and on the brain and endocrine mechanisms that subserve it. Specifically, we hypothesize that compared to controls, long-term practitioners will show increased ability to regulate thermal pain by means of meditation, as measured by self-report; and different neural patterns in response to the thermal pain stimulus and the meditation state itself. We will collect data from up to 20 long-term meditators. After collecting data from these participants, we will recruit up to 40 non-meditating control participants. Control participants will be matched to long-term meditators on age and gender. At the beginning of each visit, pain threshold will be calibrated. We will need to recruit more than 16 control subjects to ensure matching on pain threshold as well as age and gender. See Section E for detailed power analysis and justification of n. Researchers may use information learned from this study in scientific journal articles or in presentations. Clinicians may use information learned from this study to better design therapeutic intervention to alleviate pain and stress. Identifiers will be removed from all data to protect participant confidentiality. There are no direct benefits to participants. Potential risks associated with fMRI include ferromagnetic collision, neurostimulation effects, and psychological or physical discomfort. Other potential risks include discomfort of painful thermal stimulation. B. Design Up to 20 long-term meditators will be recruited by referral from long-term meditators who participated in our previous study, protocol H-2003-0475. Up to 40 control participants will be recruited through flyers, advertisements in the local newspaper, and web-based material. Interested individuals will call or email researchers. Researchers will call interested individuals to pre-screen them to ensure they meet the following criteria. 1. 25-65 years old [Reason: may affect outcome measure(s)] 2. Does not have a current problem with alcohol or non-prescription drugs [Note: we will not record answers to this question. Instead, screeners will simply make a decision to exclude if the answer is yes, note in tally form the reason for the exclusion, and then destroy all information related to this participant] [Reason: may affect outcome measure(s)] 3. Is not currently taking any prescribed psychotropic medications and has no immediate plans to start taking them [Reason: may affect outcome measure(s)] 4. Control participants must not have significant previous training or significant current practice in meditation [Reason: may affect outcome measure(s)] 5. Control participants must not have a daily practice with other mind-body techniques [Reason: may affect outcome measure(s)] 6. Is not excluded based on attached MR Screening Form (Note: this form has already been approved by the IRB for other protocols) [Reason: necessary to participate in research protocol] -8- Version 1, 2007 July 17 All participants will come for two visits. At the beginning of the first visit, participants will be screened to make sure they: (1) consent to completing study requirements and (2) meet requirements for safe scanning. First visit: Study participants will undergo a thermal pain calibration procedure; they will be instructed in the performance of the meditation states; and they will undergo a “mock” simulation scan while performing the full task. For this simulation session, participants will be brought to the neuroimaging simulator room located in the Waisman Laboratory for Brain Imaging and Behavior at the Waisman Center. They will undergo the staircase testing procedure to determine pain threshold, as described below in the section on pain stimulation. Then the meditation states will be explained. With the experienced practitioners, the investigators will confirm that they are familiar with the states; non-experienced control participants will receive a brief instruction and explanation of the states, and a sheet to take home (see description of meditation states below). Finally they will be fitted with fiber-optic goggles specifically engineered for use in the MR environment; their heads will be placed inside the head coil with padding on the top, back and sides of their head; they will be fitted with a bite bar to prevent head movement; and the bed will be positioned in the bore of the mock magnet. Throughout the mock scan, a number of different checks and inquiries will be made to assess participant comfort while in the mock scanner. The participant will rehearse the task (see below) to ensure that all contingencies and responses are well understood. This entire procedure will take approximately 1.5–2 hours. When the task has been learned, participants will be asked whether they are comfortable with the study and wish to continue. They will then be given an appointment for their scanning session. Participants will be requested to practice the meditation practices 15 minutes a day for one week prior to their scan visit. Second Visit: Participants will be screened again for safe scanning requirements. Standard MR protocol procedures to remove any ferromagnetic objects (e.g., wristwatches) will be performed. The participant will then be escorted to the 3.0 Tesla GE scanner in the Waisman Laboratory for Brain Imaging and Behavior at the Waisman Center and will be fitted with goggles for visual stimulus presentation, provided with ear protection, and positioned in the head coil and scanner. A respiratory belt will be used to measure the subjects' respiration. Final adjustments will be made to ensure that the participant can see images presented through the goggles and that they are comfortable. Given the potential for imaging artifacts due to head movement during speech, no further inquiries will be made of the participant after this point. The participant will be told that it is best for them to remain still and refrain from any head, face or jaw movements. They will be informed that while in the scanner, eye activity will be monitored using small cameras. The investigators will inform the participant of the start of each phase of the experiment as it proceeds. Standard scanner calibration will take place next followed by collection of anatomical images of the brain, which will allow for spatial identification of the activations that are measured when functional images are collected. While being set up in the scanner the participant will be given a pneumatic emergency notification button. If the participant has become uncomfortable, they can press this button and the scan as well as all experimental procedures will end. Thermal stimulation (see below) will be terminated immediately and the participant will be evacuated from the bore of the scanner within ten seconds. Participants will be told that they will receive a series of 10-second thermal stimuli preceded by 9 to 15 seconds of non-painful ramp-up heat while performing each of the two meditation states, or resting in a neutral state. -9- Version 1, 2007 July 17 Stimulus: Participants will receive 32, 10-second thermal stimuli in the scanner during the study task (16 heat and 16 warm stimuli). Prior to the study task, participants will undergo a method of staircase testing to determine their own pain threshold. The method of staircase has been previously approved in our lab (H2005-0288) and will involve 28, 8-second stimuli (14 heat and 14 warm stimuli). The description of this psychophysics task is provided below. The temperature of stimuli presented will be based on this threshold so that no participant will receive stimulation that they are not able to tolerate. These stimuli will be applied to the dorsum of their non-dominant hand. Participants will be asked to rate the stimuli on an 11-point Likert scale, ranging from 0 (no pain) to 10 (unbearable pain). A pain consistently rated as “8” will be chosen for the painful heat condition. The number and length of stimuli and method of determining individualized thermal stimuli are similar to those used in a previously approved protocol in our lab (HSC#2002-563) and have been used without incident. Stimuli will be generated by the TSA-2001, manufactured by Medoc Advanced Medical Systems. The FDA has permitted use of the device for research purposes and it has been used in research settings around the world without incident. The device has several overlapping hardware and software safety features to prevent injury, including a maximum temperature (50C.), protections against malfunction-based overheating and limits on how long stimuli can be applied (stimuli > 46C can not be applied for longer than 10 seconds). This device has been approved for use in our lab in a previous protocol (HSC#2002-563). Meditation States: The two meditation states cultivate two different cognitive/emotional strategies. 1. The participant will focus attention on an object, in this case an image on the screen, keep it on that object and bring it back to that object when distracted. 2. The participant will maintain a state of present, open awareness, neither focusing on any particular object nor allowing the mind to wander. When the mind wanders or becomes focused, the participant will return to the state of open awareness. More detailed instructions can be found in the attached meditation instruction sheet. C. Subject population and recruitment Because there are only a small number of people who have sufficiently long-term meditation experience with the practices we are investigating, recruitment of practitioners is mainly accomplished through word-ofmouth and referral from previous participants. Control participants will be recruited for the study through flyers specifically designed for the current project (see attached) which will be posted publicly around Madison. Participants may also be recruited through newspaper advertisements in local papers, targeted DoIT email lists of faculty and staff, public email lists such as Craig’s List, and email or web-based forums specific to ethnic groups. Recruitment materials will use the same text as the attached flyer. Interested participants will contact the study coordinator by email or telephone. Study coordinators will screen participants by telephone. Participants will be offered $75 for completion of the study. Control participants will have the opportunity to receive an additional $50 bonus if their brain activation in areas associated with attention are in the top four of all controls. If a participant terminates participation after the first visit, he or she will be paid $25. After volunteers have been contacted for participation they will come to the first study visit, in which the experiment will be explained in detail and consent procedures implemented D. Minimal risk This study involves some risk for study participants. See “Risks” section below. - 10 - Version 1, 2007 July 17 E. Sample size justification We will recruit up to 20 long-term practitioners in an effort to collect good data on 16. We will recruit ageand gender-matched control participants who will undergo thermal pain threshold calibration. Control participants must also be matched with practitioners on thermal pain threshold; therefore we will recruit up to 40 controls until we have found enough who match the practitioners on all relevant characteristics. We now justify the sample size of 16. The effect of meditation training was estimated in a previous study (Brefczynski-Lewis et al. 2007). In this study, we showed that the state of compassion meditation enhances the brain response to a stressful auditory sound in several brain regions and that this effect was modulated by the degree of training to meditation. This study thus suggests that meditation training can modulate the brain response to sensory events and supports our main assumption for this new fMRI study. We used this study to estimate the possible impact of mental training on sensory (thermal) stimulation. In the inferior parietal cortex, for instance, the difference in the mean percentage of increase from neutral to meditation between expert meditators and novices was 0.18% with a standard deviation of 0.15%. If it is assumed that the between-subjects variance measured in this study is a good approximation of the population variance in this area, then one can estimate that the effect size d is equal to 1.125. From there, one can estimate that the degree of noncentrality δ for a conservative sample size of N=16 is δ=d*sqrt(N/2)=3.2. Then, from statistical tables, one can estimate that the power is 0.81 using a two-tailed test at α=0.02 and for δ=3.2. From this analysis we predict that a conservative sample of N= 16 participants in each group will enable rejection of the null hypothesis at an alpha of 0.02. F. Risks Potential risks associated with fMRI include ferromagnetic collision, neurostimulation effects, and psychological or physical discomfort. Other potential risks include discomfort of painful thermal stimulation. G. Benefits There are no direct benefits to participants. H. Consent Procedures Written informed consent will be obtained from all participants using either the “long-term practitioner” consent form or the “control participant” consent form. (see attached consent forms). I. References Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A. 2007 - 11 - Version 1, 2007 July 17