Rock Density Lab
advertisement

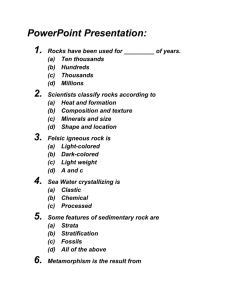
ROCK DENSITY / SPECIFIC GRAVITY (70 points) INTRODUCTION: How does metamorphism change the density of a rock? Density is one of the most fundamental properties of geological materials. Density is helpful in identification of rocks and minerals. Density is defined as mass per unit volume (ie. grams / cubic centimeter). It helps to describe how packed together molecules of a substance are. In this lab what you are actually measuring is specific gravity. Specific gravity is a dimensionless number (no units), and is the ratio of the weight of a rock to the weight of an equal volume of water. This is a way of determining density based upon the buoyancy of a substance in water. For our lab purposes, there is no difference between the two. PURPOSE: 1. To measure, record, and analyze data correctly 2. To compare features of various rock types and how they formed 3. To compare the densities of sedimentary and metamorphic rocks MATERIALS: Single pan balance (with loop or hook to suspend objects from the bottom of the pan) Collection of different rock types and sizes (about golf ball size) 1000ml Beaker filled to 750ml of water String (for sling to hold rock samples suspended in the water) Stand for the balance (for safety and stability place books on the base!) Figure 1: Balance set up. PROCEDURE: 1. Record the type of rock for your first sample (if known), on the DATA TABLE. 2. Set up the balance and construct a sling from the fishing line to suspend the rock samples in the beaker of water. Zero your balance with the string sling hanging from it. (Important!) (see figure 1). 3. Find the weight of the first sample (in air), by placing the sample on the balance and using the method prescribed for the type of balanced used. Record the mass on the DATA TABLE to the nearest 0.1 grams. 4. Suspend the first sample from the fishing line sling with the rock completely immersed in the beaker of water (again see figure 1). Find the weight of the sample (in water), and record on the DATA TABLE to the nearest 0.1 grams. 5. Calculate the loss of weight in water using the following: weight-weight in water = weight loss. Record this on the data table under weight loss 6. Now calculate the density of the sample to the nearest .01 g / cm3, and record your answer on the DATA TABLE: Use the following formula. Density = Rock weight in air = Weight loss in water Mass Volume Quick explanation: Water has a density of 1 g/cm3, thus it buoys up anything within it by 1 gram per cubic centimeter of displacement. Because of this, the weight in air minus the weight in water is equal to the volume of the rock sample in cm3. 7. REPEAT STEPS 1-6 for each rock sample. 8. Create a bar graph of rock density for analysis. (10 points) Use proper interval, range, and labels. Use of color should serve a purpose i.e. metamorphic rock one color, parent rock another color. DATA TABLE: Density of Rocks Sample 1. 2. 3. 4. 5. 6. 7. 8. Rock type (if known) (16 Points) Rock weight in air (g) Rock weight in water (g) Weight loss (g) Rock density (g / cm3) Questions: (write Q & A in 2 columns) (12 points) 1. Compare and contrast granite and gneiss. 2. How does the grain size of sandstone change during metamorphism? 3. What textural differences do you observe between shale and slate? 4. What are some reasons your rock densities might not be the same as other students in the room. Comment on error that causes inaccuracy. How does this error cause change in the anticipated result? Be Specific. Conclusions: (respond in paragraph form) (6 sentence minimum) (30 points) 1. Why does the color of a sedimentary rock change during metamorphism? 2. Discuss the rock cycle. Pick one of your rock samples and discuss how it would move through the cycle describing the processes and the changes it would undergo. 3. Compare the densities of shale and slate, sandstone and quartzite, limestone and marble. Which of these are metamorphic? Sedimentary? Is there a relationship between density and rock type? Discuss this relationship using detail, examples, and data.