Project Proposal dra..
advertisement

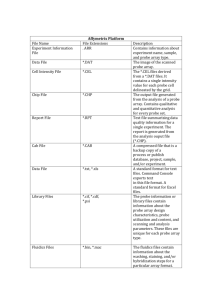
Project Proposal draft 3 June 30, 2006 Title of Project: Developing an Optimized qPCR for Borrelia lonestari Name: HaoQi (Esther) Li Objective: The purpose of this project is to find and optimize the probe for detecting the Lyme-causing bacteria Borrelia lonestari. Justification: Lyme disease is caused by bites from a Borrelia-bacteria-carrying tick. The symptoms, such as rash, fever, fatigue, etc. resemble those of common ailments, thus making the diagnose of Lyme disease more challenging. Borrelia lonestari is a recently discovered Lyme-causing spirochete, i.e. a spiral shaped bacterium. Its vector is the lone star tick, Amblyomma americanum, commonly found in the southeastern U.S. Like the other strands of the gram-negative Borrelia, B. lonestari has two flagella that can facilitate the corkscrew movements of the spirochete, making the disease more easily to be spread in a host’s body. If this experiment shall succeed, the synthesized Beacon probe will be very specific for the detection of B. lonestari’s flagellin genes, providing a more efficient tool to diagnose Lyme disease from the A. americanum vector. Description: Testing Design: Using programs ClustalW and GeneDoc, the flagellin sequence of B. lonestari is aligned and compared to the sequences of other Borrelia strands. Manually find areas highly unique for B. lonestari as possible regions for the Beacon probe and primers. Then test the feasibility of the proposed probes with Beacon Designer 4.0 and use the program to optimize the length and locations of the primers. Send the request to Sigma-Genosys to synthesize the probe. Finally test the probe using real-time PCR (r-t PCR), i.e. quantitative PCR (qPCR), to determine the sensitivity of the probe. If the results are not desired, then draw conclusions and modify the probe on Beacon Designer and repeat the process again. Controls: negative control = no DNA. Positive control = B. lonestari flagellin DNA positive 1. clean room (no DNA allowed) negative control 2. dirty hood negative control 3. positive control The sensitivity of the probe is done by using the positive control with the known 10 3 copies of B. lonestari flagellin DNA along with other Borrelia, Rickettsia, and other bacteria samples: No. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Strain 1B. recurrentis 2B. coriaceae 3B. burgdorferi 4B. afzelii 5B. hermsii 6B. garinii 7B. duttoni 1R. prowazekii Breinl 2R. prowazekii Ananiev 3R. typhi Musibov 4R. canada 5R. rickettsii R 6R. rickettsii 364-D 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 7R. conorii 8R. parkeri 9R. montana 0R. slovaca 1R. sibirica 2R. japanica 3R. akari 1Escherichia coli 2Proteus mirabilis OXK 3Salmonella enterica 4Legionella pneumophila 5Francisella persica 6Bartonella quintana 7Bartonella vinsonii 8Neorickettsia sennetsu 9Neorickettsia riticii 0Orientia tsutsugamushi 1Staphylococcus aureus 2Corynebacterium sp Using the online BLAST (Basic Local Alignment Search Tool) program, the targeted probe is compared to all other sequences in the gene bank to insure its uniqueness. And indeed, this expectation has been proven true. Quantitative goal: The probe should detect at least 10 copies of Borrelia lonestari flagellin DNA per μl. In addition the probe should not detect any copies of Borrelia lonestari in the non-B. lonestari samples. Materials: In addition to the basic material such as test tubes, micropipettors, lab coats, gloves, cleaning liquid, biohazard bags, test tube racks, micropipettor tips, dNTP, MgCl2, qPCR buffers, H2O etc. The programs ClustalW and GeneDoc are used for sequence alignment and comparison. The program Beacon Designer 4.0 is used for optimizing the possible probe and primers. Company Sigma-Genosys for synthesizing the Beacon probe. The program and machines of Smart Cycler 2.0d are used for running the qPCR. Costs: The estimated coast for each sample reaction is $1.3 for the buffer, the tube, the micropipette tips, etc. The cost of the probe synthesis by Sigma-Genosys after a discount will be $150. Procedures: Preparation for qPCR analysis in the clean room (i.e. no DNA) Safety: Put on lab coats and gloves, set up biohazard bags in the plastic enclosed workspace. Prepare test tubes, test tube racks, micropipetters (10μl, 20μl and 100μl), micropipetter tips of different sizes Vortex and centrifuge the reagents and place them in a test tube rack and start transferring materials by specific μl amounts. Clean up the workplace with DNase and RNase cleaning solution Continued in the dirty hood… Add specific concentration of each DNA sample into special qPCR tubes with lenses (for the fluorescent light detection) After vortex and centrifuging, place the samples inside SmartCycler II qPCR machine and start the SmartCycler computer program to receive data from the machine. Clean up workspace and wait for the results on the computer. Testing: For cross reactivity testing, 100pg of DNA is used in each reaction, which is about 10 4 – 105 copies in each reaction For the positive control, 103 copies of DNA is used in the reaction Trials: 3 trials Results I will measure and collect the data by using the Smart Cycler 2.0d which yields results like: Analysis: I would look at the logarithmic curve of the fluorescence detected and if everything goes according to the plan and the probe is specific, only the positive sample that contains B. lonestari would have a curve over the threshold. The threshold is arbitrary however the logarithmic curve is the strong indication of a positive B. lonestari DNA sample. The sensitivity of the probe can be seen from the x-axis of the graph, which indicates the number of DNA amplification cycles. A more sensitive probe emits fluorescence in earlier cycles. If the probe will be specific and sensitive, then future scientists can use the probe designed in this experiment for medical purposes of detecting Lyme disease caused by the lone star tick. Limitations: The biggest concern, besides limiting the probable contamination, is that there will be some undesired bonding by the probe unpredicted by the Beacon Designer 4.0 program so that the experiment fails to run. If time will be limited, not all 32 strands of DNA will be tested, however, it is crucial that all the other Borrelia sequences are tested before the experiment is over. http://www.sigmaaldrich.com/Brands/Sigma_Genosys.html Mentor signature: References Cutler, S. J., Moss, J., Fukunaga, M., Wright, D. J., Fekade, D., & Warrell, D. (1997, October). Borrelia recurrentis characterization and comparison with relapsing-fever, lyme-associated, and other Borrelia spp. International Journal of Systematic Bacteriology, 47(4), 958-96p8. Similar methods Kelly, D. J., Richards, A. L., Temenak, J., Strickman, D., & Dasch, G. A. (2002, June). The past and present threat of Rickettsial diseases to military medicine and international public health. Clinical Infectious Diseases, 34(Suppl. 4), S145-S169. Meri, T., Cutler, S. J., Blom, A. M., Meri, S., & Jokiranta, T. S. (2006, July). Relpsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators c4b-binding protein and factor H. Infection and Immunity, 74(7), 4157-4163. Nice introduction on Borrelia Probe based quantitative PCR. (2006). Quantitative PCR. Retrieved June 21, 2006, from Sigma-Aldrich Co. Web site: http://www.sigmaaldrich.com/Area_of_Interest/Life_Science/Molecular_Biology/PCR/ Quantitative_PCR.html What is lyme disease? (2006). Lyme disease. Retrieved June 29, 2006, from American Lyme Disease Foundation Web site: http://www.aldf.com/lyme.shtml