Hoh Fuk Tong College
advertisement
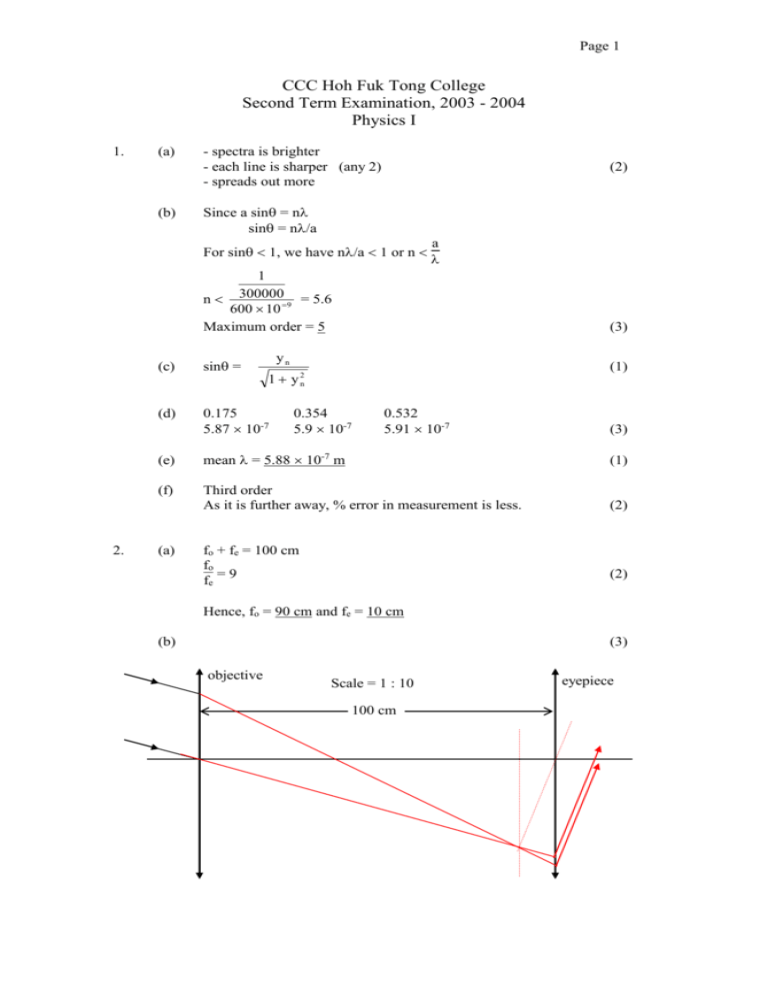
Page 1 CCC Hoh Fuk Tong College Second Term Examination, 2003 - 2004 Physics I 1. (a) (b) - spectra is brighter - each line is sharper (any 2) - spreads out more (2) Since a sin = n sin = n/a For sin 1, we have n/a 1 or n a 1 n 3000009 = 5.6 600 10 Maximum order = 5 2. (3) yn (c) sin = (d) 0.175 5.87 10-7 (1) 1 y 2n 0.354 5.9 10-7 0.532 5.91 10-7 (3) (e) mean = 5.88 10-7 m (1) (f) Third order As it is further away, % error in measurement is less. (2) (a) fo + fe = 100 cm fo fe = 9 (2) Hence, fo = 90 cm and fe = 10 cm (b) (3) objective Scale = 1 : 10 100 cm eyepiece Page 2 (c) minimum diameter of eyepiece fe =f diameter of objective o minimum diameter of eyepiece (d) (e) 3. (a) (2) The eye-ring is the best position where the eye should be placed because it collects as much light as possible from that passing through the telescope. The diameter of the eye-ring = 0.44 cm (3) Advantage: It gives an erect image/It is shorter in length. Disadvantage: The view angle is smaller/The image is dimmer. (2) (i) To ensure that the elastic limit is not exceeded. (1) (ii) slope of graph = E (iii) (b) 10 = 4 90 = 0.444cm (i) (ii) 26.5 - 10.5 -1 -1 7.4 - 3 = 3.64 cm kg = 0.0364 m kg F/A Fl Lgl L l = e/l = = = e gA eA eA 1 l 1 2.00 = slope g A = 0.0364 10 2 6.0 10 4 4 9 -2 = 1.9 10 N m (3) F 15 10 = 5.3 108 N m-2 A= 4 2 6.0 10 4 1.9 10 9 E v= = = 1300 m s-1 ρ 1100 d 10 t = v = 1300 = 0.0075 s (2) (3) F Breaking stress ( A ) remains unchanged, i.e. 5.3 108 N m-2 15 kg 4 = 2m m = 1.875 kg Therefore (3) Page 3 4. (a) (i) (ii) W W = - P dV = - P (Vf – Vi) (for constant pressure) = - (5 105) (3 10-3 - 6 10-3) = 1500 J (2) Vf = - P dV = - nRT ln(V ) (for constant temperature) i Vf 6 10-3 = - PB VB ln(V ) = - (5 105) (3 10-3) ln( ) 3 10-3 i = -1040 J (2) (b) 3 EA TA Since E = 2 nRT, E = T B B 4500 1200 EB = 600 , EB = 2250 J Since B and C are on the same isotherm, EC = EB = 2250 J (c) 3 Since E = 2 nRT 3 At A: 4500 = 2 n (8.31)(1200) n = 0.3 mole (d) (i) (ii) 5. (a) (3) (2) U = W + Q (2250 – 4500) = 1500 + Q Q = -3750 J U = W + Q 0 = -1040 + Q Q = 1040 J A En = - n2 = -6.98 (4) A En+1 = - (n + 1)2 = -3.10 (n+1)2 6.98 n2 = 3.10 n = 2 A Hence, - 22 = -6.98 A = 27.92 (b) 27.92 En = - n2 E1 = -27.92 eV E2 = -6.98 eV E3 = -3.10 eV E4 = -1.745 eV…………… (3) E4 E3 E2 E1 (2) Page 4 (c) E1,2 = -6.98 – (-27.92) = 20.94 eV 26.0 eV E1,3 = -3.10 – (-27.92) = 24.82 26.0 eV E1,4 = -1.745 – (-27.92) = 26.175 26.0 eV The highest energy level = 3 Energy of bombarded electron = 26.0 – 24.82 = 1.18 eV (d) E = (4) hc 24.82 (1.6 10-19) = (6.626 10-34) (3 108) = 5 10-8 m (500 Å) It is ultra-violet. (e) (2) Since an atom will only go to an excited state if it absorbs the right amount of photon energy completely. As there is no exact energy difference corresponding to the photon energy, the atom will not be excited. (2) END OF PHYSICS I