845 A pulsed laser emits light at a wavelength of 694
advertisement

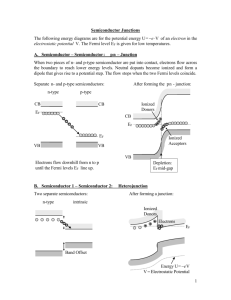
Raffles Institution (J C) JC2 H2 Physics 2009 Physics Tutorial : 20 LASERS AND SEMICONDUCTORS Self-Check Questions : Part A : LASERS. S1. What does LASER stand for ? Light Amplification by Stimulated Emission of Radiation. S2. List the characteristics of laser light. Laser light is highly coherent, unidirectional (or collimated) and monochromatic. S3. Explain the difference between spontaneous emission and stimulated emission of radiation from atoms ? In spontaneous emission, a photon is emitted by an atom randomly and in any direction, without any external stimulation, whereas in the case of stimulated emission, an incoming photon, whose energy is exactly equal to the excitation energy of the atom, induces the excited atom to fall to a lower energy level and releases a photon in the process. This photon released is similar to the one which induces its emission. The two photons are emitted at the same time and in the same direction. S3. What is meant by a ‘population Inversion ‘ of atoms ? Population inversion is a situation in which for a given sample of atoms of the same element, there are more atoms in the excited state than in the ground state. S4. Why is a ‘population Inversion ‘ necessary in the construction of a laser ? ‘Population inversion’ of atoms in the metastable state is necessary in order that the photons produced by the first stimulated emission events can further trigger more excited atoms to produce identical photons. In this way, a chain reaction of stimulated emissions can occur to build up a laser beam of a sufficiently high intensity. S5. Explain the use of the two mirrors placed at the ends of a laser. The mirrors at the ends of the laser face each other and are parallel. They reflect the photons produced by stimulated emission back into the lasing medium and along the axis of the laser cavity so that more stimulated emissions can occur to produce the laser beam. One mirror is totally reflecting while the other is partially reflecting in order to allow the laser beam to emerge. 1 Part B : SEMICONDUCTORS S6. Name two examples of Intrinsic Semiconductors. Silicon and germanium S7. What is meant by ‘doping’ of a semiconductor ? Hence how may ( I ) a p – type semiconductor be obtained ? ( 2 ) an n – type semiconductor be obtained ? Give one example for each of the doped semiconductors. Doping is a process in which a small number of atoms of an element of valency 3 or 5 are added into the lattice of silicon (or germanium) with the purpose of increasing the number density of free charge carriers which will ultimately increase the electrical conductivity of the semiconductor. Example : An impurity concentration of 1 part in 108 increases the conductivity of germanium by a factor of 12 at room temperature. A p-type semiconductor would be formed if silicon is doped with aluminium (valency 3) and an n-type semiconductor would be obtained if silicon is doped with phosphorous (valency 5). In the p-type material, the number density of holes is significantly increased, thus making holes the majority carriers. In the n-type material, the number density of electrons is increased and these are the majority carriers. One way of understanding how hole number density outnumbers electron number density (as is the case for p-type material) is that since aluminium atoms have only 3 valence electrons to form covalent bonds with 4 neighbouring silicon atoms, there will be a missing electron in one of the 4 covalent bonds. This missing electron is a hole which can accept an electron from another covalent bond. Thus compared with pure silicon, there are now more holes in the lattice. Another way of understanding this effect is by using the band theory --- see S9. S8. Compare the electrical conductivities of metals and semiconductors using the ‘Band-Theory’ of energy levels. In a metal, the highest occupied energy band is not full. Hence, electrons in this band can easily gain just a little energy to occupy other empty levels within the same band. In other words, the electrons can easily move through the lattice when a potential difference is applied. However, in a semiconductor such as silicon, the highest occupied band (called the valence band) is completely filled at low temperatures. Thus electrons within the same band cannot move. The next available band, called the conduction band has plenty of empty levels and is separated from the VB by an energy gap of only1.2 eV. Electrons at the top of the VB can easily gain 1.2 eV of energy to cross the gap over to the CB. In doing so, an equal number of holes will be created in the VB. Thus both electrons in the CB and holes in the VB contribute to current flow if a potential difference is applied across the semiconductor. The conductivity of a semiconductor therefore depends very much on the number density of charge carriers (includes both holes and electrons) and this number naturally increases with temperature. As the number density of charge carriers (electrons) in a metal is very high (~1029 m—3 for copper) compared with that in an intrinsic semiconductor (~1016 m—3 for silicon ), the electrical conductivity of metals is much higher than that of semiconductors. 2 S9. In terms of ‘Band Theory’, why is the electrical conductivity of a doped semiconductor much higher than that of a pure semiconductor ? In the case of n-type silicon, the presence of dopant atoms introduces donor states within the energy gap lying very close to the bottom of the conduction band. Electrons from the donor states easily gain the small amount of energy required to go into the CB. (example: Ed is 0.05 eV below the CB for silicon doped with phosphorous). At room temperature, typically all the electrons from the donor states are in the CB. Thus the number density of charge carriers is significantly increased compared with that of the intrinsic semiconductor. Similarly in the case of p-type semiconductor, the presence of dopant atoms introduces acceptor states into the energy gap and they lie very close just above the top of the valence band. Electrons from the top of the VB readily gain the small amount of energy required to go into the acceptor states, thus creating holes in the VB. As a result, the number density of charge carriers in the p-type material increases and this explains why the conductivity increases. S10. What is meant by the ‘depletion layer (or zone) ’ in a p-n junction ? Explain its formation. When a p-type semiconductor is jammed against an n-type semiconductor forming a plane junction, electrons from the n-side close to the junction will diffuse over to the pside because there are few electrons there. In so doing, positive charge accumulates on the n-side. Similarly, holes from the p-side close to the junction will also diffuse to the nside, causing negative charge to appear on the p-side. The electrons diffusing from the n-side can recombine with holes on the p-side and results in a loss of free electrons and free holes (the charge carriers in both types of materials) over a certain width at the p-n junction. The appearance of positive charge on the n-side and negative charge on the pside produces an electric field directed from the n-side to p-side and we can associate this electric field with a potential difference across the junction. The diffusion of charge carriers across the junction will eventually stop when the potential difference is high enough to prevent further movement of charge carriers across the junction. The region over the p-n junction in which there are no free charge carriers is called the depletion layer (or zone). S11. Why does a p-n junction conduct electricity predominantly in only one direction ? In forward bias, since the p-side is made more positive than the n-side, the height of the contact potential V0 is reduced and hence more of the majority charge-carriers can now surmount this smaller barrier. Thus the diffusion current increases markedly and this explains why current flows from the p-side to the n-side during forward bias. In reverse bias, the p-side is made negative with respect to the n-side. This causes the contact potential to increase and no electrons can move from the n-side to the p-side. In other words the majority charge carriers cannot flow through the junction. The minority charge carriers can flow across the junction, but because their number is very small, the resulting current is negligible. Thus the p-n junction only conducts current predominantly in one direction, that is; from the p-side to the n-side during forward bias. 3 S12. Sketch the Current – Voltage Characteristics of a typical diode. I/mA 4 3 2 1 -1 Reverse-bias - 0 1 Forward-bias + 2 V/V Discussion Questions : Part A : LASERS [Data given : Speed of light = 3.0 x 108 m s—1 , Planck constant = 6.6 x 10—34 Js] 1. A pulsed laser emits light at a wavelength of 694.4 nm. The pulse duration is 12.0 ps, and the energy per pulse is 0.150 J. (a) What is the length of the pulse? (b) How many photons are emitted in each pulse? Solution : (a) (b) Length of pulse = (12.0 x 10—12 ) x 3.0 x 108 = 3.60 mm number of photons = 0.150 x 694.4 x 10—9 / (6.6 x 10—34 x 3.0 x 108 ) = 5.26 x 1017 4 2. In a ruby laser, light of wavelength 550 nm from a xenon flash lamp is used to excite the chromium, Cr3+ ions in the ruby from ground state E1 to state E3. In subsequent de-excitations, laser light is emitted. Which of the following statements regarding this laser is incorrect? 2.25 eV E3 1.79 eV E2 Optical pumping (550 nm photons) Ground state E1 A E3 cannot be the metastable state because, if it is, then there will be no net production of light when equilibrium is reached, since stimulated absorption and stimulated emission will then occur at the same rate because the number of electrons in E3 and E1 will be the same at steady state. B E3 is the metastable state because, having a longer lifetime than a normal excited state, the metastable state allows the accumulation of excited electrons, resulting in population inversion and net light production. C E2 is the metastable state because it is not subjected to stimulated emission caused by the 550 nm photons used in optical pumping, and so allows the accumulation of excited electrons to achieve population inversion. D The transition from state E2 to E1 produces the laser light. 2. B -- Option A explains the answer. (You can also refer to the discussion on two-level, three-level and four-level laser systems found at the end of the Appendix of the lecture notes for a clearer understanding). 3. Which of the following statements concerning a laser system is false ? A An external energy source is needed to create population inversion. B The laser beam produced is coherent and of a single wavelength. C By changing the reflective coefficient of the partially reflecting mirror, the intensity of the laser beam can be varied. D High-end laser systems can produce perfectly collimated beams, i.e. beams that would not spread. 3. D -- Diffraction will cause a spreading of the light beam. 5 Part B : SEMICONDUCTORS 4. The diagram shows the two highest energy bands of 3 materials. (II) (I) (III) Which of the following is a possible material for each set of energy bands shown ? 5. (I) (II) (III) A Germanium Plastic Silver B Tungsten Gold Silicon C Wood Silicon Copper D Nickel Germanium Water Which of the following statements is correct ? A An n-type semiconductor has a net negative charge. B A p-type semiconductor conducts through holes only. C A net current always flows across the p-n junction due to the junction electric field. D The junction electric field always points from the n-type to the p-type regions across the depletion region. 5. D – The n-side is positive and the p-side is negative. 6 6. When a photon enters the depletion zone of a p-n junction, the photon can scatter from the valence electrons there, transferring part of its energy to each electron, which then jumps to the conduction band. Thus, the photon creates electron-hole pairs. For this reason, the junctions are often used as radiation detectors, especially in the x-ray and gamma - ray regions of the electromagnetic spectrum. Suppose a single gamma - ray photon of frequency 1.6 x 1020 Hz transfers its energy to electrons in multiple scattering events inside a semiconductor with an energy gap of 1.1 eV, until all the energy is transferred. Assuming that each electron jumps the gap from the top of the valence band to the bottom of the conduction band, find the number of electron-hole pairs created by the process. Solution : The number of electron-hole pairs = energy of gamma ray photon/energy gap = (6.6 x 10—34 )(1.6 x 1020 )/(1.1 x 1.6 x 10—19 ) = 6.0 x 105 7. A potassium chloride crystal has an energy band gap of 7.6 eV above the topmost occupied band, which is full. Is this crystal opaque or transparent to (a) light of wavelength 140 nm ? and (b) violet light ? Solution : (a) For light of wavelength 140 nm, the photon energy = (3.0 x 108 x 6.6 x 10—34 )/(140 x 10—9 x 1.6 x10—19 ) = 8.84 eV Since this is higher than the band gap energy of 7.6 eV, the photons can be absorbed by the material ( that is, electrons from the VB can absorb the photons and get promoted into the CB). Thus the material is opaque to light of wavelength 140 nm. (b) For violet light, a typical value of its wavelength is 400 nm. The photon energy is = (3.0 x108 x 6.6 x10—34 )/(400 x 10—9 x 1.6 x10—19 ) = 3.09 eV Since this is less than the band gap energy, the light will not be absorbed. The material is thus transparent to violet light. ---------------------------------------------------- END----------------------------------------------------- 7


![Semiconductor Theory and LEDs []](http://s2.studylib.net/store/data/005344282_1-002e940341a06a118163153cc1e4e06f-300x300.png)

