Experiment 2: Ratios - Breck School Science
advertisement

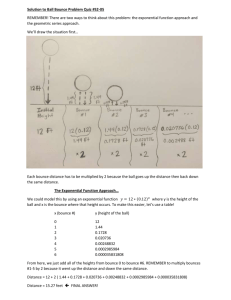
Bouncy Ball Mark Ledford and Nolan O’Brien 2003 Abstract In our project, we tested the bounce height of balls made from sodium alginate and sodium borate. We wanted to make the bounciest ball possible, and in doing so, we tested the variables such as: ratio, diameter, different types of glue-white glue, rubber cement, glue stick- temperature-ranging from-5C to 30C. When we tested each of the variables, we bounced the balls created and calculated the average bounce height as to see which variable created the bounciest ball. We found that the ratio of 10 g of glue, 5 g of sodium borate, and 5 g of water had the highest bounce height of 36.52 cm. For the sodium alginate, we used the ratio of 10 g of glue, 5 g of sodium alginate, and 5g of water, had the next highest average bounce height of 16.25 cm. The smallest diameter for each of the polymer balls gave the highest bounce. The sodium alginate ball with diameter of 2.25 cm had an average bounce height of 40.31 cm. The sodium borate ball with a diameter of 1.81 cm had an average bounce height of 37.25 cm. The smallest diameter for each of the polymer balls created the highest bounce. The sodium alginate ball with diameter of 2.225 cm had an average bounce height of 40.31 cm. The sodium borate ball with diameter of 1.81 cm had an average bounce height of 37.25 cm. The sodium borate did not solidify with either he rubber cement, or the glue stick, but when we used sodium alginate, the glue stick produced the highest average bounce, which was 38.63 cm. The staring temperature for the sodium alginate was –2.5° C, and the ending temperature was 7.7° C. The staring temperature for sodium borate was –5.0° C and the ending temperature was 7.4° C. For both of the polymer balls, temperature had a direct relationship with bounce height, because as the temperature went up, so did the height of bounce. Overall, based on each of the experiments, the sodium alginate proved to bounce higher than the sodium borate ball. Introduction Introduction The purpose of this project was to create the bounciest putty possible from sodium alginate and sodium borate. To create the bounciest putty possible, we conducted four different experiments by adjusting the following variables: ratio (sodium alginate/borate to glue; water to sodium alginate/borate), temperature, size and types of glue: Prang glue stick, white glue and rubber cement. We hypothesized that size would have an indirect relationship with height, and that the putty made from sodium borate would bounce higher than the putty made from sodium alginate. We also hypothesized that temperature would have an indirect relationship to height, that the best bouncing ratio would be of 10.0g glue, 2.5g polymer and 5.0g water, and that the most effective glue, increasing the bounce of the ball, would be rubber cement. Polymer Background A polymer is a long change of molecules that contain repeating structural units (monomers) that link together with strong spines of single bonded carbon atoms (1). A monomer is a type of molecule that chemically links together with other monomers, creating a polymer. Monomers have at least one double bond, which is broken during polymerization. When writing a monomer, the “n” symbolizes the number of monomers within the polymer (2). Isobutylene, which has a monomer of C4H8, is a common polymer that is normally used in adhesives (2). Polymers are the foundation of all adhesives. There are two types of adhesive polymers: the homopolymer and the copolymer. The homopolymer consists of only one type of monomer while the copolymer are comprised of more than one monomer (1). All different types of adhesives have to go through a fundamental change in order to form bonds. Each adhesive has its own unique way of converting from a liquid or a workable solid into a hard and cohesive solid (1). There are three different types of conversion properties of adhesives: evaporation, chemical conversion and thermal conversion. In evaporation adhesives that contain water cure to solids as the water evaporates into the atmosphere. In a chemical conversion, adhesives that contain components that produce a chemical reaction turn the adhesive into a cured solid. A reaction can be triggered by moisture, polyurethane glue, a catalyst or by epoxy (1). A thermal conversion is dependent on adhesives that only work when heated. Glues that can be heated to promote workability are known as thermosetting adhesives. When glue polymerizes, the polymers become organized into a solid matrix (1). The adhesives that form molecular strands between the monomers become connected, which is known as cross linking. Adhesives that cross link are generally more durable and water resistant than those that do not (1). In our project, we are using sodium alginate and sodium borate as a main ingredient of our polymer bouncy balls. Sodium alginate is the sodium salt of algenic acid. The chemical formula for sodium alginate is (C6H7NaO6)n (3). Sodium alginate occurs as a white to yellowish brown filamentous, grainy, granular or in powdered form. It is most commonly used as a stabilizer, a thickener, a gelling agent and an emulsifier. Sodium borate is a highly toxic substance. Its chemical makeup is Na2B4IO7(4). It is a white, gray, bluish or in greenish white streak crystals. It is odorless and is stable under ordinary use and storage. Sodium Borate has a molecular weight of 381.37 grams/mole. Elmer’s white glue is bonded by solvent evaporation. The solvent in Elmer's glue is water. When the water evaporates, the polyvinyl acetate latex, which formula is CH3COOCH:CH2 that has spread into a material's cracks forms a flexible bond (5). The Glue stick used in our project was made by the Prang Corporation. Glue stick acts in the same way as normal white glue, with the exception that the company has eliminated water from the glue so there is none absorbed in the atmosphere(5). Rubber cement is C4H6, and its solvent is made of 87% hexane and n-hexane, and 13% non-hazerdous ingredients (6). Its incompatibles are strong oxidizing materials, liquid chlorine, concentrated oxygen, and sodium hypochlorite. Rubber cement is highly toxic, because it contains ingredients that are toxic and should only be used with sufficient ventilation like a fume hood (6). In our project, we used rubber cement, white glue, and a melted form of glue stick as our adhesives to bond with sodium borate and sodium alginate. The different adhesives that we used vary in bond strength, and so, the glues will affect the bounce height (7). Altering different ratios will force glues to change the way it bonds, and thus affect the bond strength and height of bounce (1). Some water-based glues freeze between 25°C30°C, and many of these glues can be made ineffective, and unable to form bonds if frozen at low temperature, while some glues will only work at high temperatures (1). For our internet interview with Mr. Chong (8), a scientist in the adhesive department from Dixon Ticonderoga Corporation, Q: How long have you been working for Dixon Ticonderoga A: For about two years now. Q: Based on your products of white glue and glue sticks, do you know of any difference in bond strength between these two adhesives? A: No, I don’t believe so. Q: If we were to change the temperature of an already cured adhesive, what do you think would happen to the strength of the bond in the adhesive once it has come back to room temperature? A: Well freezing or cooling certain types of glues ruins the actually adhesive, while heating the glue will only break bonds made, but they would be made again once the temperature returns to normal. The freezing might have an negative effect on the glue, but the heating of the glue would not have any effect on the bonds. Q: Do you know if the size of the objects that adhesives are bonded to have any correlation with the strength of the bond? A: Yes, although it is harder to apply to smaller objects, when adhesive is applied to a smaller rather tan large object, the bond is prone to be stronger and to last longer. Procedure Experiment 1: Polymer Study 1. We used the 10.0 g of glue, 2.5 g of polymer and 10.0 g of water ratio for the model of this experiment. By following the ratio, we simply plugged in different polymers into the ratio to see which one, after formed into a ball, would bounce the highest. We compared the bounces of each polymer by giving it a number between 1 and 10. The higher the number means the better the bounce the polymer ball produced. 2. First we plugged sodium alginate into the ratio from the above step. Then, after we formed the ball, we dropped it three times to test the bounce by softly nudging the ball off of the table, insuring a constant height of the drop. Although the ball did not bounce, it was able to form into a solid. Therefore, we gave sodium alginate a 4. 3. We then plugged the following polymers into the ratio from step 1: sodium borate, adipoyl chloride, calcium chloride, guar gum and latex. By following the process of step 2, we were able to determine and compare each of the effects of polymers on bounce height. We gave adipoyl chloride, calcium chloride, guar gum and latex all 1’s. This is because none of these polymers solidified (formed into a solid) and could not be bounced. However, the sodium borate had a very small bounce. Because of its bounce, we gave sodium borate a number 6. 4. We looked back at our data and decided that we would use sodium alginate and sodium borate for the rest of our project because they worked best out of all the other polymers. Experiment 2: Ratios Part 1: Sodium Alginate 1. First we mixed together 10.0 g of glue, 2.5 g of sodium alginate and 5.0 g of water. 2. We formed the product from step 1 into a ball and then set it aside to dry. 3. We propped a meter stick against the side of the table to measure the bounce of the sodium alginate ball. Then we put the ball from step 2 at the edge of the table and softly nudged it over the edge. We then measured the height of the bounce by using the meter stick. Then we repeated step 3 two more times with the same ball to insure accuracy. We found that the average bounce of this sodium alginate ball with this ratio was 25.44 cm. 4. We repeated steps 1-3 with these different ratios of sodium alginate and water; 2.5 g of sodium alginate, 10.0 g of water; and 5.0 g sodium alginate and 5.0 g water. For the ratio of 2.5 g sodium alginate and 10.0 g of water, we got an average bounce of 20.33 cm. For the ratio of 5 g sodium alginate and 5.0 g water, we got an average bounce of 26.25 cm. 5. We determined that the ratio of 2.5 g sodium alginate and 5.0 g water, which had an average bounce height of 26.25 cm, would be the best ratio to use for experiments 3,4 and 5. Part 2: Sodium Borate 1. We repeated steps 1-3 with sodium borate in lieu of the sodium alginate. We determined an average bounce height of 22.72 cm. 2. We repeated steps 1-3 with these different ratios of sodium borate and water; 5.0 g sodium borate, 5.0 g water; 5.0 g sodium borate and 2.5 g of water. For the 5.0 g sodium borate and 5.0 g water ratio, we got an average bounce height of 36.52 cm. For the 5.0 g sodium borate and 2.5 g of water ratio, we found an average bounce height of 18.69 cm. 3. 3. We then compared the different bounce of the ratios and determined that the ratio of 5.0 g sodium borate and 5.0 g water, which had an average bounce height of 36.52 cm, would be the best ratio to use for experiments 3,4, and 5. Experiment 3: Temperature Part 1: Effects of Lower Temperature 1. We made sodium alginate and sodium borate balls with the best bouncing ratios described in experiment 2. The ratio for the alginate ball was 10.0 g of glue, 2.5 g sodium alginate and 5.0 g water. The best ratio for the sodium borate ball was 10.0 g of white glue, 5.0 g sodium borate and 5.0 g water 2. We inserted thermometers into each of the balls. 3. We placed both balls, with the thermometers attached to them, into the freezer and chilled them to the temperature of the freezer, which caused the sodium alginate ball to chill to –5.0°C and the sodium borate ball to chill to –1.5°C. 4. 4. After the balls had been chilled, we tested the bounces of the balls in two minute intervals as their temperature’s returned to room temperature. 5. 5. We recorded the bounce for each trial and found that, as the temperature of the balls increased, the height of the bounce also increased. Experiment 4: Diameter/Proportions Part 1: Sodium Alginate 1. We made the sodium alginate balls in this experiment referring to the best bouncing ratio described in experiment 2. The ratio for the alginate ball was 10.0 g of glue, 2.5 g sodium alginate and 5.0 g water. 2. We split the ratio, from the above step, of each ingredient in half and created a new putty. After the putty was formed, we measured and recorded the diameter of the ball by using a vernier caliper. We found the diameter to be 2.25 cm. We then dropped the ball three times and recorded the bounces. We ended with an average bounce of 40.31 cm. 3. We then doubled the ratio of each ingredient from step 1, created the putty, and measured and recorded the diameter with a vernier calipur. We found the diameter to be 3.95 cm. We then dropped the ball three times and recorded the bounces. We ended with an average bounce of 28.25 cm. 4. We observed our data. We found that bounce is indirectly proportional to height. As the ball got bigger, we found that the height became smaller. Part 2: Sodium Borate 1. We repeated steps 1-4 with sodium borate. The best ratio for the sodium borate ball was 10.0 g of white glue, 5.0 g sodium borate and 5.0 g water. For the doubled ratio, we ended with a diameter of 3.75 cm and an average bounce height of 31.75 cm. And for the halved size we had a diameter of 1.81 cm and an average height of 37.25 cm. Experiment 5: Varied Glues Part 1: Sodium Alginate 1. We used the best bouncing ratio of sodium alginate, which was 10.0 g of glue, 2.5 g sodium alginate and 5.0 g water, as the model ratio for this experiment. However, we substituted these different glues into the ratio: white glue, glue stick, and rubber cement. 2. We melted the Prang Glue Stick. We then substituted the melted glue stick into the ratio from step 1 by replacing the regular white glue. 3. We formed the putty, dropped it three times and recorded the bounce results. We found that we had an average bounce height of 38.63 cm. 4. We then substituted rubber cement into the ratio from step 1 by using it as the glue. After forming the putty into a ball and dropping it three times, we found the average bounce height to be 36.07 cm. 5. We compared the average bounces of white glue, glue stick and rubber cement and determined that the Prang Glue Stick produced the highest bouncing ball of putty with an average bounce height of 38.63 cm. Part 2: Sodium Borate 1. We repeated steps 1-3 with the best bouncing sodium borate ratio, which was 10.0 g of glue, 5.0 g sodium borate and 5.0 g water. However, we found that the sodium borate was unable to form into a ball and therefore unable to bounce with either rubber cement or Prang Glue Stick. Results Quality (Bounce Height) Figure 1: Pre-Lab Investigation on Varied Polymer Effectiveness 7 6 5 4 Quality 3 2 1 ex La t G ua r G um or id e hl C al ci um C A di po yl C hl or id e e or at B od iu m S S od iu m A lg in a te 0 Polymer (Name) Figure 1 shows the quality of different polymers in which they are ranked on a scale of 1 to 10, 1 being the worst and 10 being the best. The polymers ranked at 1 are all at the same height because none of those polymers solidified (formed into a solid) and could not be bounced. We gave sodium borate a six and sodium alginate a four because the bounce height of the sodium borate ball was higher. Figure 2: Different Ratios of Sodium Borate Ball 12 Amount (g) 10 8 Ratio 1 Ratio 2 Ratio 3 6 4 2 0 Glue (g) Water (g) Sodium Borate (g) Water (g) Ingredient (Name) Figure 2 shows how much of each ingredient was put into each ratio of the sodium borate ball. The first ratio consists of 10g of glue, 2.5 of sodium borate and 5g of water. The second ratio consists 10g glue, 5g sodium borate, and 5g of water. The third ratio consists of 10g glue, 5g of sodium borate and 2.5g of water. Refer to Figure 3 for correlation with bounce height. Figure 3: Different Ratio's Average Bounce Heights for Sodium Borate Average Bounce Height (cm) 40 35 30 25 Average Bounce Height (cm) 20 15 10 5 0 1 2 3 Ratio Number Figure 3 shows the average bounce height for the three different ratios for sodium borate shown in figure 2. The first ratio had an average height of 22.72 cm. The second ratio had an average height of 36.53 cm, while the third ratio had an average height of 18.96 cm. Figure 4 shows the 3 different ratios used to make the sodium alginate balls. The first ratio consists of 10 g of glue, 2.5 g of sodium alginate, and 5 g of water. The second ratio consists of 10 g of glue, 2.5 g of sodium alginate, and 10 g of water. The third ratio consists of 10 g of glue, 5 g of sodium alginate, and 5 g of water. Figure 5: Different Ratio's Average Bounce Heights for Sodium Alginate Average Bounce Height (cm) 30 25 20 Average Bounce Height (cm) 15 10 5 0 Ratio 1 Ratio 2 Ratio 3 Ratio Figure 5 shows the average height bounce for the three different ratios of sodium alginate balls made shown in figure 4. The average bounce height for the first ratio was 25.42 cm; the average bounce height for the second ratio was 20.33 cm; the average bounce height for the third ratio was 26.25 cm. Figure 6: Effect of Diameter on Sodium Alginate Bounce Height 45 Width/Height (cm) 40 35 30 25 Diameter (cm) Bounce Height (cm) 20 15 10 5 0 1 2 3 Different Sizes Figure 6 shows the effect of diameter on bounce height of a sodium alginate ball. With a diameter of 2.95 cm, the ball bounced 26.25 cm; with a diameter of 2.25, the ball bounced 40.31 cm; with a diameter of 3.95 cm, the ball bounced 24.92 cm. Figure 4: Different Ratios of Sodium Alginate Ball 12 Amount (g) 10 8 Series1 Series2 Series3 6 4 2 0 Glue Water Sodium Alginate Ingredient (Name) Water Figure 7: Effect of Diameter on Sodium Borate Bounce Height 40 Height/Width (cm) 35 30 25 Diameter (cm) Bounce Height (cm) 20 15 10 5 0 1 2 3 Differnet Sizes Figure 7 shows the effect of diameter on bounce height of a sodium borate ball. With a diameter of 2.41 cm, the ball bounced 36.52 cm; with a diameter of 1.81, the ball bounced 37.25 cm; with a diameter of 3.75 cm, the ball bounced 31.75 cm. Figure 8: Effect of Different Glues on Bounce Height 45 Bounce Height (cm) 40 35 30 25 Sodium Alginate Sodium Borate 20 15 10 5 0 Rubber Cement Prang Glue Stick White Glue Type of Glues Figure 8 shows the effect of different glues on the height of bounce for sodium borate and sodium alginate. The sodium borate did not solidify with the rubber cement or the prang glue stick, there for the values for that in figure 8 are zero. The sodium borate had an average bounce height of 37.25 cm when the white glue was used. The sodium alginate ball had an average bounce height of 30.07 cm with the rubber cement, 38.63 with the prang glue stick, and 40.31 cm with the white glue. Figure 9:Effect of Temperature on Bounce Height for Sodium Borate 30 Bounce Height (cm) 25 20 15 Sodium Borate 10 5 0 -10 -5 0 5 10 Temperature (°C) Figure 9 shows the effect of temperature on the bounce height of the sodium borate ball. At the starting temperature of –5.0° C, the bounce height was 2.7 cm. As the temperature continued to rise, the height of bounce did as well, until it 5.7° C when the height of bounce was at 22.5. The temperature rose 2°C after that point, but the height of bounce only rose to 23.9 cm. Figure 10: Effect of Temperature on Sodium Alginate Bounce Height 35 Bounce Height (cm) 30 25 20 Sodium Alginate 15 10 5 0 -5 0 5 10 Temperature (°C) Figure 9 shows the effect of temperature on the bounce height of the sodium alginate ball. At the starting temperature of –2.5° C, the bounce height was 4.3 cm. The graph continued to rise exponentially until the ball was at a temperature of 5.0° C, when the height of bounce stayed at a height of about 30.1 cm. Conclusion The purpose of this project was to make the bounciest ball possible from sodium alginate and sodium borate. We conducted four different tests to see which variable added had the greatest effect on bounce height. The four tests we did were ratio, size, varied glue, and temperature. We found that the ratio of 10 g of glue, 5 g of water, and 2.5 g of sodium alginate created the bounciest ball for the sodium alginate ball. The average bounce height for sodium alginate with this ratio was 26.25 centimeters. We then found that the ratio of 10 g of glue, 5 g of sodium borate, and 5 g of water created the bounciest ball for the sodium borate ball. The average bounce height for the sodium borate with this ratio was 36.52 g. After finding the results of this lab, we continued research of our project, but we only used the optimal ratios as to optimize the bounce height for each ball. We multiplied the best ratio we found in the ratio lab by 1, 0.5, and by 2. We then took the diameter to see if there was any correlation between size and bounce height. For the sodium alginate, we found that the ball with a diameter of 2.25 cm had the highest average bounce, which was 40.31 cm. For the sodium borate, we found that the ball with the diameter of 1.81 cm had the highest average bounce, which was 37.25 cm. For both of the polymers, when the ratio found in the ratio lab was multiplied by 0.5, the ball created had thee highest average bounce height. We used rubber cement and prang glue stick as our variables to test if the type of glue had any effect on bounce height. When we tested the sodium borate with both of the glues, we found that the product did not solidify, therefore making the results for that part of the lab not applicable. For the sodium alginate, we found that the average bounce height when rubber cement was used was 36.07 cm, and when the glue stick was used, the average bounce height was 38.63 cm. The staring temperature for the sodium borate was –5.0° C and the bounce height at that temperature was 2.7 cm. The ending temperature was 7.4° C and the bounce height at that temperature was 23.9 cm. With the sodium alginate, the starting temperature was – 2.5° C and the bounce height at that temperature was 4.3 cm. The ending temperature was 7.7° C and the bounce height at that temperature was 30.3 cm. For both the sodium alginate and the sodium borate balls, the temperature had a direct relationship with the bounce height. Based on our results, we found that our original hypothesis which was that the size would have an indirect relationship with height, temperature would have an indirect relationship with bounce height, the rubber cement would create the bounciest ball, that the 10 g of glue-2.5 g of polymer-5 g of water ratio would create the bounciest ball and that the ball made from sodium borate would bounce higher than the ball made from sodium alginate. Based on our results, we found: that our hypothesis for the size lab was correct; the hypothesis for the temperature lab was incorrect; the hypothesis for the ratio lab was incorrect; that the hypothesis for the varied glue lab was also incorrect. The overall study showed that in all of our experiments, the sodium alginate bounced higher than the sodium borate, thus refuting out overall hypothesis. For further study, we suggest that students, instead of using sodium alginate, or sodium borate, that they use a different polymer. One could work with another polymer that currently does not bounce, and work with it until they came up with a way in which it does bounce. Questions that could be answered by further research include: Is it a coincidence that out of all the polymers we used, the ones that actually bounced were sodium based? Why did the size have an indirect relationship with bounce height? Bibliography 1) W. Young, The Glue Book. (Taunton Press, Newtown, CT, ed.1, 1998) p. 169. 2) L. Freun, The Real World of Chemistry. (Kendall/Hunt Publishing Company, Dubuque, IA, ed.6, 2002) p. 203-205 3) Compendium of Food Additive Specifications (1997) see; http://www.fao.org/docrep/W6355E/w6355e0x.htm 4) Strategic Services Division (2003) see; http://www.sefsc.noaa.gov/HTMLdocs/SodiumBorate.htm 5) How Stuff Works (2003) see; http://science.howstuffworks.com/question695.htm 6) Rubber Cement (1996) see; http://www.camd.lsu.edu/msds/c/cement_rubber.htm#Physical 7) Access Science (2003) see; http://www.accessscience.com/serverjava/Arknoid/science/AS/Encyclopedia/0/01/Est_011050_printable.html 8) P.Chong, personal communication. 4 Apr 2003.