Chapter 12 – Clouds & Precip
advertisement

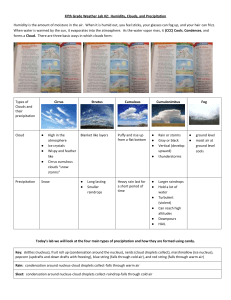
Eth Sci Chapter 12 – Clouds & Precip What is the most impor gas in the atmosphere? ______________ Water vapor I. Changes of State Water vapor defined as an Oxygen and nitrogen also change state from gas to liquid to solid, but at much lower temps than we see on earth…wtr vpr is unique as a gas in moving from gas to liq to solid at the press and temp we see at earth surface Change of state requires heat absorption or release.. Heat is measured by calories – 1 cal = amt heat required to raise temp of 1 g of water 1o C latent heat (“hidden” heat) example is when heat is taken up to melt ice to water…all heat is taken up in breaking solid xl structure to liquid…when all ice melted, then T of water strts to rise, but not before all ice melted latent heat important source of much energy, for example clouds, tstorms, trop storms, hurricanes Fig 12.1 shows specifics of heat absorption/release - Latent heat of evap…600 cal needed to convert 1 g wtr to wtr vpr Interesting pt here..higher -Latent heat of condensation is the reverse, where 600 cal given off…this energy used in producing heavy weather, violent storms - - latent heat of melting … 80 cal req to produce 1 g of water from solid latent heat of fusion …80 cal released during freezing…reverse of above sublimation – direct conversion of solid to gas – dry ice a good example of this…another example is unused ice cubes which eventually sublimate in a freezer..680 cal absorbed by a gram of ice for it to sublimate reverse of sublimation is “deposition”…also can be frost..household example is frost in an older freezer – 680 cal released when frost is formed Eth Sci wk 12 p.2 I. Humidity – water vapor in the air 2 types of humidity – specific, relative relate these back to concepts of sautration and vapor pressure.. saturation – putting as much wtr vpr into air as it can hold w/out precipitating vapor pressure – that part of atmos press that is due to water vapor, say above a body of wtr at saturation, the no. of molecules evaporating = no. of molecules condensing temp complicates situation So specific humidity = wtr vpr wt / total wt of mass, And relative humidity = actual wtr vpr content / potential wtr vpr content The 2 factors that can change rel humidity – 1. add or subtract water Fig 12.3 2. change temp Fig 12.4 if hold wtr content constant, rise in temp will lower rel humidity (because poten wtr vpr content goes up) drop in temp will increase Fig 12.5 Another impor concept – dew point Is the temp to which air of measuring humidity psychrometer used, which is two thermometers, one dry –air, the other wet-air. Table used to compare the two readings Earth Sci wk 12 p. 3 II. Basis of cloud formation: Adiabatic cooling Now look at wtr vpr role in weather and fm of clouds… Fog/dew vs clouds..all 3 require saturation Radiation cooling causes dew and fog fm. Clouds different… Air that is compressed has temp increase (molecules closer together) Air that is decompressed expands and cools These temp changes are not assoc w/heat loss or gain, but with pressure changes…known as adiabatic changes As air rises it cools adiabatically, as it drops it warms ..rate of temp change is known as dry adiabatic rate if air unsaturated If air rises and cools enough it will reach dew point, and after this as it continues to rise, its latent heat of condensation will be released as vapor cools and condenses Fig 12.7 ….this is mechanism of cl III. Stability of air Why air rises sometimes, or why cloud sizes differ, has to do with air stability Stability is function of temp. …cool air will sink according to its density, reach stable position …but warm air will rise, like hot air balloon and be unstable stability prevails when the environmental lapse rate is less than the wet adiabatic rate Fig 12.8, Fig 12.9 the rising air being forced upward still would rather sink downward note the flat clouds, trying to spread out Unstable air is when envir lapse rate is greater than the dry adiabatic rate Fig 12.10 Earth Sci wk 12 p.4 Rising air would just as soon keep on rising….note how clouds billow upward…. Stability and daily weather Stable air sometimes is forced to rise, even though it is relatively cool..when this occurs, clouds are pretty flat, have little precip Unstable air has towering clouds, heavy precip For example, on overcast drizzly day, stable air has been forced up Contrast this to big upward-growing clouds, probably unstable air IV. Processes that lift air orographic lifting Fig 12.11 “mountain lifting” ….precip example – Olympic Mts in often see rainshadow, abs frontal wedging…due to wedge of cool air that warm air overrides can result in both stable and unstable air Fig 12.13 V. convergence – where air mases flow together Condensation and Cloud Formation Condensation defined- when water vpr in air changes to a liquid This produces dew, fog, or clouds Usu occurs because temp cools to dew pt, but sometimes because water is added to air Usu need a surface for condensation Ex dew on grass, car wind Or in case of clouds, need condensation nuclei (bits of dust) At start of condensation, w.v. becomes millions of tiny droplets (or ice crytals) suspended in air Earth Sci wk 12 p.5 Cloud classification Defined as “visible aggregates of minute droplets or xls of water” - Classif based on: form height - Form – 3 types Fig 12.14 cirrus – high white thin, ptches, sheets, or wispy cumulus – individual “globs” stratus – sheets or layers…no individual globs Other component of cloud classif - height 3 types – fig 12.15 high – above 6000 m (about 20,000 ft): - cirrus – thin, delicate mare’s tails - cirrostratus – thin sheets - cirrocumulus-fluffy masses middle (2000 to 6000 meters) “alto” is the prefix,like diff between alto and soprano voices in choir..alto is middle female voice - altocumulus – globby clouds that are larger, denser than cirrocum - altostratus – white to grayish sheets low – - stratus – uniform, fog-like, cover much of sky - stratocumulus – scalloped bottoms, rod-like - nimbostratus – “rain” + “sheets” , major rain makers, forced ascent of stable air clouds with low bases, high tops…all assoc with unstable air cumulus can grow to cum VI. Fog Defined- a cloud with its base at or near the ground Dff betw fog and other clouds are in Method of fm Place of fm Earth Sci wk 12 p.6 Clouds are result of adiabatic cooling, but fog forms from radiation cooling or mvmt of air over a cold surface Fog also can form by addition of wv to air (evaporation fog) A. fog caused by cooling 1. advection fog – Fig 12.16 due to warm air over cool surface,T drops below dew pt 2. radiation fog – Fig 12.17 cool calm nights when earth losing heat through radiation.. air in contact with ground cooled below dew point..pockets of fog form in low spots like river valleys 3. upslope fog – humid air moves upslope, cools adiabatically B. fog caused by evaporation forms from cool air moving over a warm surface (usu. water), happens because water evaps from warm surface up into cloud and causes saturation 1. steam fog - rising water vapor immediately recondenses – looks like steam rising from lakes and rivers 2. frontal fog – warm air lifted over cold air, rain develops, then rain evaporates to produce fog VII. Precipitation What causes it? How it forms…millions of cloud droplets (about 1/8 diam of human hair) coalesce into bigger droplets, large enough to fall 2 mechs proposed to explain this – 1. ice-crystal process..based on observation that precip often forms at temps below freezing in high lat clouds…those droplets that make contact with a solid particle are the ones that freeze remaining droplets are supercooled, but are still liquid, not ice Earth Sci wk 12 p.7 this sets stage for precip..ice xls collect water, grow into snowflakes. They start to fall, get bigger as they pick up the supercooled could droplets that freeze on the flakes… then when these particles get low enough to warm up, the snowflakes change to water, fall rest of the way as rain 2. collision-coalescence process start with condensation nuclei…large droplets form, then collide with smaller droplets that coalesce with the big ones, ultimately the droplets become big and heavy enough to fall forms of precipitation- 1. rain - drops of water at least 0.5 mm in diam, rarely bigger than 5 mm 2. drizzle- drops of water less than 0.5 mm in diam 3. snow – in form of ice xls known as snow flakes, size shae, form controlled by temp at formation.. Snow (cont) Snow formed at low T is light, fluffy, due to low moisture content of air.. this is the powder that skiers love…. Above a temp of – 5o C (about 22 deg F), snow is wet , tangled clumps with high moisture content, ideal for snowballs 4. sleet – small particles of ice that are essentially melted snow which froze..the melted snow falls through a cold layer of air just above ground,and becomes sleet 5. glaze is technical term for freezing rain..conds same as for sleet, except the melted snow (supercooled) doesn’t freeze until it hits something solid, like a car or tree branch or power line 6. hail – hard pellets of ice, often concentric layers showing progressive growth from small nuclei to large ball largest to date rept’d in Kansas, how big?? How heavy?? What town did I live in when a hailstorm ruined my roof? Hail produced in cumulonimbus , storm clouds, big updrafts, ice particles form, drop, get picked up, get bigger (new layer of ice), fall again, get picked up again, get a new layer, process repeats until it finally falls to grd 7. rime – deposited by supercooled fog