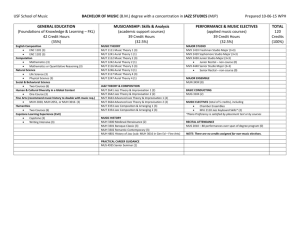
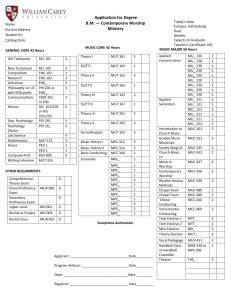
jws-prot.21284
advertisement

Statistical-thermodynamic analysis of mutagenetic effects on HX
We have shown previously that the Ghx value of a particular residue j
produced by a mutation can be interpreted from a statistical-thermodynamic point of
view according to the following equation 1:
K mut
j ,ex
hx, j
Ghx, j RT ln
RT ln
wt
j ,nex
K hx, j
(1)
G{ j ,ex} G{ j ,nex}
wt
mut
where K hx
, j and K hx, j are the equilibrium constants of the conformational processes
opening residue j to HX for the WT and mutant protein, respectively. j,ex and
j,nex are average cooperativity factors between the residue j and the interaction
affected by the mutation for the HX-competent {j,ex} and the HX-protected {j,nex} submut
ensembles. Finally, G{mut
j ,ex} and G{ j ,nex} represent the energy shifts produced by the
mutation in each sub-ensemble. The net energy shift is indicative of the effectiveness in
the propagation of the local energy perturbation, i.e., of the co-operativity between
residue j and the position of mutation.
It is useful to define a marker residue, m, as a residue undergoing HX only in
states with the position of mutation unfolded and protected from HX in states with the
position of mutation folded. Thus, the HX of the marker residue fully senses the local
stability changes produced by the mutation. The Gibbs energy shift produced by the
mutation in the HX-competent ensemble of the marker residue, G{mut
m,ex} , will be equal
mut
to both the local energy perturbation, Glocal
, and the Gibbs energy shift of the
1
globally unfolded ensemble, G{mut
U } . Similarly, the Gibbs energy shift in both the HXmut
protected ensemble, G{mut
m,nex} , and the native ensemble, G{N } , will also be equal
assuming that the probability that the position of mutation unfolds independently of the
marker residue is very small. Therefore, for the marker residue the experimental
Ghx,m value will be equal to the change induced by the mutation in the global
unfolding Gibbs energy, GU.
Dividing equation 1 by either Ghx,m or GU:
Ghx, j
Ghx,m
mut
G{mut
j , ex} G{ j , nex}
mut
G{mut
m, ex} G{m, nex}
Ghx, j
GU
mut
G{mut
j , ex} G{ j ,nex}
(2)
Gmut
Gmut
U
N
Except for residues exchanging in very low-energy states, usually undetected in
standard HX measurements, the HX-protected ensemble {j,nex} of most residues
resembles very much to the native ensemble, {N}, since highly-structured states of the
protein are by far the most probable ones in both ensembles. Therefore,
Gmut
Gmut
j , nex
N :
Ghx, j
Ghx,m
Ghx, j
GU
mut
G{mut
j , ex} G{N }
mut
G{mut
m, ex} G{ N }
(3)
This analysis is similar to the mutagenesis analysis of folding transition states of
proteins 2. The interpretation of equation 3 is complicated by the presence of Gmut
N in
both the numerator and the denominator (ground-state effects) that usually arise from
conformational changes induced in the native ensemble by the mutation. Nevertheless,
it is possible to find circumstances for a simple interpretation of these fractions:
2
a) Mutated residue k in a highly-stable region: The probability of the mutated
residue being unfolded in the {N} ensemble is very low and, therefore, ground-state
0 and equations 2a and 2b simplify to:
effects are very small. Gmut
N
Ghx, j
Ghx,m
G{mut
j ,ex}
G{mut
m,ex}
G{mut
j ,ex}
Gmut
U
(4)
This ratio can be interpreted as the average relative extent in which the
interactions affected by the mutation are disrupted in the conformational ensembles
rendering each residue of the protein exposed to the solvent.
b) Mutated residue k in a low-stability region and residue j in the same lowstability region or in a high stability region: Most states exposing residue j to HX will
Gmut
Gmut
also have the mutated residue unfolded. Accordingly, Gmut
j , ex
m, ex
U ,
and:
Ghx, j
Ghx,m
Ghx, j
GU
1
(5)
c) Both residues j and k in different low-stability regions: The mutation may
induce a redistribution of probabilities of local fluctuations affecting these low-stability
regions, giving rise to local conformational changes and energy shifts in the {N}
ensemble. If these ground state effects are important, they may spoil the meaning of
these ratios. Nevertheless, this would affect only to the interpretation of co-operative
effects between different low-stability regions of the protein.
Similar results to those of the previous treatment can be reached by another way:
The average cooperativity factors in equation 1 can be sub-partitioned in states with the
mutated residue (residue k) folded (kf) and unfolded (ku):
3
mut
j ,ex F j ,ex( kf ) F j ,ex( ku) ·local
(6a)
mut
j ,nex F j ,nex( kf ) F j ,nex( ku) ·local
(6b)
In these equations the quantities F are the fractions of each sub-ensemble with either
residue k folded or unfolded. We have also assumed that only states with k unfolded
mut
mut
have their energy affected by the local energy perturbation ( Glocal
). A
RT ln local
similar partitioning has been made elsewhere in a computational study of protein
cooperativity 3. Combining equations 6a and 6b:
e
Ghx, j
RT
mut
local
1·F j ,ex( ku) 1
mut
j ,nex local
1·F j ,nex( ku) 1
j ,ex
(7)
And reordering equation 7:
F j ,ex( ku)
e
Ghx, j
RT 1
mut
local
1
e
Ghx, j
RT ·F
(8)
j ,nex( ku)
Now we can consider the same circumstances as above:
a) Residue k at a high-stability region: For any residue j, most states protecting it
from HX will have also k folded and, therefore, Fj,nex(ku) 0 , and equation 8 simplifies
to:
F j ,ex ( ku)
e
Ghx, j
RT 1
mut
local
1
e
e
Ghx, j
Ghx, m
RT 1
RT 1
e
e
Ghx, j
GU
RT 1
(9)
RT 1
This quantity does not differ much from equation 4. Indeed, using the expansion: ex =
1 x + high order terms, for relatively small energy perturbations the errors introduced
by ignoring the high order terms in both the numerator and the denominator tend to
compensate in the fraction. Accordingly:
4
F j ,ex( ku)
Ghx, j
Ghx,m
Ghx, j
GU
(10)
b) Residue k in a low-stability region and residue j in the same low-stability region or in
a high stability region: Most states exposing residue j to HX will also have residue k
unfolded. Accordingly, F j,ex(ku) 1 .
c) Both residues j and k in different low-stability regions: In this case equation 8 cannot
be simplified. The second term on the right hand side of the equation depends on the
probability of independent unfolding of j and k and on the concomitant the ground-state
effects as discussed above.
Using these fractions together with the experimental equilibrium opening
constants, Kop, for the WT protein, we can discern between the probabilities of HXcompetent states for residue j that are either cooperative or non-cooperative with the
position of mutation:
Pj ,ex ( ku)
Pj ,ex ( kf )
K op, j
1 K op, j
K op, j
1 K op, j
·F j ,ex ( ku)
(11)
·F j ,ex ( kf )
References
1.
Casares S, Sadqi M, Lopez-Mayorga O, Martinez JC, Conejero-Lara F.
Structural cooperativity in the SH3 domain studied by site-directed mutagenesis
and amide hydrogen exchange. FEBS Lett 2003;539(1-3):125-130.
5
2.
Matouschek A, Kellis JT, Serrano L, Fersht AR. Mapping the Transition-State
and Pathway of Protein Folding by Protein Engineering. Nature
1989;340(6229):122-126.
3.
Hilser VJ, Dowdy D, Oas TG, Freire E. The structural distribution of
cooperative interactions in proteins: analysis of the native state ensemble. Proc
Natl Acad Sci U S A 1998;95(17):9903-9908.
6