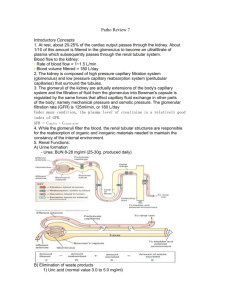
Kidneys, Ureters, and Suprarenal Glands
advertisement

Renal #9 Tues, 03/18/03, 11am Dr. Mallet Jennifer Uxer for Jennifer Derby Page 1 of 9 Glomular Filtration Note: The beginning of this lecture wrapped up the introductory lecture from Monday. Some figures have been included in this scribe because that was the best way to describe what was said in class. Other figures were not included because word descriptions were sufficient. Be sure to look at these in the powerpoints. Clearance Clearance describes how effectively the kidneys remove a substance from the bloodstream and excrete it in the urine A. Calculation of Clearance Clearance of substance X = concentration of X in urine * urine volume concentration of X in plasma CX = (UX * V)/PX Example: urea Purea = 20 mM Uurea = 400 mM V = 1 ml/min Curea = (400 mM * 1 ml/min)/20mM = 20 ml/min Note the units: volume per unit time (ml/min) B. GFR can be determined from clearance of certain compounds Requirements: compound must be freely filtered, but neither secreted nor reabsorbed If these requirements are met: The amount filtered = amount excreted, then the GFR = the clearance: GFR * PX = UX * V Rearranging, we find: GFR = (UX * V)/ PX = CX In reality, this isn’t true for any compound in the body. C. Inulin clearance Inulin clearance = GFR Inulin isn’t found in the body; it’s a fructose polysaccharide that must be infused. So, it’s not practical for clinical use. Inulin: freely filtered, neither reabsorbed, secreted nor metabolized Amount inulin filtered per unit time = amount excreted per unit time. GFR * Pin = Uin * V Rearranging: GFR = (Uin * V)/Pin = Cin D. Creatinine Use of inulin is cumbersome; creatinine is produced endogenously (muscle metabolite), so no infusion is needed. It behaves a lot like inulin. All filtered creatinine is excreted. Some is secreted in the proximal tubule causing an overestimation of GFR because the numerator is a bit too high. Glucose and other stubstances causes over estimation of creatinine in the plasma. So the 2 overestimations cancel out. The two sources of error nearly cancel out, so Ccrt = (Ucrt x V)/Pcrt ~GFR This is difficult to use clinically because you have to depend on patient compliance to collect all their urine over a period of time. E. Pcrt monitors renal function (see B&L Fig. 40-12, p. 688) Pcrt (plasma creatinine concentration) is inversely proportional to GFR Renal #9 Tues, 03/18/03, 11am Dr. Mallet Jennifer Uxer for Jennifer Derby Page 2 of 9 Theoretically, if GFR falls to 25% of normal (there’s ¼ of the renal mass remaining), Pcrt should increase fourfold In reality, inverse relationship isn’t perfect: – Differences in lean muscle mass—more lean muscle = more creatinine. – Compensatory increased proximal tubule secretion of creatinine when the kidneys begin to fail. This decreases the plasma [creatinine]. Useful: long-term monitoring of renal function. If you have diabetic patients, you’d like to know when they begin to experience kidney failure. Taking blood samples over time will provide this information: if GFR falls, creatinine begins to accumulate in the blood. Pcrt vs. GFR: the ideal and the real—see ppts for the plots. These are graphs of the theoretical plasma creatinine vs. the glomerular filtration rate in a healthy, young male. This is about 1mg/dL of creatinine per 180L/day (GFR) As renal function declines and GFR declines, creatinine will go up. In reality (right plot), the plot is shifted to the left and down, relative to the theoretical plot, due to the compensatory secretion in the proximal tubule. Additionally, the values are scattered for different people. The trend is the same, though. If there’s an increase in creatinine, you can identify renal failure. For example, an increase from 1 to 3 mg/dL indicates a tremendous decline in GFR and a loss of about 90% of the nephrons. F. PAH clearance estimates renal plasma flow—the total volume of plasma flow through the kidneys Para-amino hippuric acid (PAH): – Is an artificial drug and is a small molecule. – Freely filtered – Avidly secreted in proximal tubule – If the PAH concentration is low, the nephrons can remove all of the PAH from the kidneys. PAH is completely cleared from peritubular capillaries when the concentration of PAH is low (i.e. when it is NOT saturated) For the exam, assume that the PAH clearance = renal plasma flow because there’s a LOW concentration of PAH. RPF ~ CPAH = (UPAH * V)/PPAH SUMMARY: Key information from session 1 Nephron segments and where they are located Renal microcirculation is unique: 2 sets of arterioles and capillaries in series Sympathetic innervation provides for arteriole constriction and renin secretion Basic processes of urine formation: glomerular filtration, tubular reabsorption and secretion, urinary excretion Clearance: (U * V)/P This can be used to estimate GFR and renal flow. Renal #9 Tues, 03/18/03, 11am Dr. Mallet Jennifer Uxer for Jennifer Derby Page 3 of 9 Case: acute renal failure A 23 y.o. woman with no history of chronic illness is referred to your nephrology clinic. Last month she had a high fever for 3 days, but had been feeling better until 5 days ago, when she noticed her urine was tea-colored. Subsequent urinations have been infrequent and darker brown, and her ankles have become swollen, prompting a visit to her F.P. Physical exam is remarkable for periorbital edema and 4+ edema of the lower extremities. Plasma chemistries reveal creatinine of 3.5 mg/dl (normal 0.6-1.4 mg/dl) and BUN (blood urea nitrogen) of 70 mg/dl (normal 11-23 mg/dl). Why are BUN and creatinine elevated in this patient? Because her renal function is down; there’s a drop in GFR. Why has she developed edema? Due to the drop in GFR, the kidneys are not excreting a certain amount of water. So, she’s retaining too much water, and it’s causing an expansion of the extracellular fluid. This is common in post streptococcyl glomelular nephritis. The strep infection damaged her glomelular capillaries and filtration membrane. So, she has also begun to have hematuria— blood in the urine. I. II. III. Glomerular Filtration Objectives Unique structure and properties of glomerular membrane. It is very permeable to water allowing lots of plasma to be filtered in a short time. Filterability of plasma constituents—small molecules freely filtered; large molecules remain in blood vessels Physical forces (Starling forces, like colloid osmotic pressure) that determine GFR Physiological mechanisms for modifying GFR Autoregulation of renal blood flow and GFR to maintain them at fairly constant levels Pathophysiology of proteinuria (protein in the urine) Filtrate Glomerular filtration: first step in urine formation Plasma is filtered, due to high hydrostatic pressure, from glomerular capillaries into Bowman’s capsule Normally, glomerular filtrate is essentially free of blood cells, proteins. Only a small amount of protein is found in the filtrate unless there’s pathology. Glomerular filtrate, which initially resembles plasma, is heavily modified as it passes down the nephron segments. Urine is very different from glomerular filtrate (plasma) Glomerular membrane: A. A molecular sieve—a screen Allows free passage of small stuff: water, small solutes (glucose, amino acids, electrolytes): concentrations are the same on both sides of membrane Renal #9 Tues, 03/18/03, 11am Dr. Mallet Jennifer Uxer for Jennifer Derby Page 4 of 9 in the plasma and in the glomerular filtrate. So concentration of glucose in Bowman’s capsule should be exactly the same as the concentration of glucose in the plasma. Passage of large molecules (proteins) and formed elements is impeded. Their size and charge prevents large molecules from passing through. Only very small amounts of protein are filtered into Bowman’s capsule. Most are reabsorbed by endocytosis in the proximal tubule. Large amounts of protein in urine (proteinuria) indicate renal injury Molecular weight and charge affect filterability [substance] in Bowman’s Capsule/[substance] in plasma --1 = freely filterable and there’s the same concentration on both sides of the membrane. --Smaller the number means that less substance crosses the membrane. B. Structure of glomerular membrane The behavior of the membrane reflects its structure. Three distinct layers: 1. Fenestrated (Swiss cheese) capillary endothelium: highly permeable to water, dissolved solutes 2. Glomerular basement membrane contains collagen, glycoproteins that contain anionic charges—negative charges. These negative charges repel other negatively charged molecules. 3. Podocyte epithelium: slit pores between podocytes restrict large molecules In an electron micrograph of glomerular filtration membrane (B&L Fig. 406, p. 682) the podocyte epithelium, basement membrane, endothelial fenestrae and slit pores can been seen. A slit diaphragm can be found between the slit pores. This diaphragm is a lattice layer of protein (think French windows). These small holes restrict the protein movement. So, proteins and large molecules are prevented from crossing the membrane by 2 methods: 1. sterically—the size of the molecule that crosses the slit diaphragm is limited and 2. electrostatically—negative molecules are repelled. Glomelular capillaries are a clinched fist that’s been pressed into a water balloon—Bowman’s capsule. Impact of electrostatic charge on filterability of large molecules (B&L Fig 40-15, p.690). Dextrans are large, artificial compounds about the size of proteins. Renal #9 Tues, 03/18/03, 11am Dr. Mallet Jennifer Uxer for Jennifer Derby Page 5 of 9 Freely filtered— small ions Ions with a positive charge are more filtered than those that are neutral or negatively charged. Larger ions Ions with a negative charge are least filterable. Most proteins at physiologic pH are polyanionic (negatively charged). IV. Factors affecting glomerular filtration A. Physical forces GFR is remarkably high (c. 125 ml/min, 180 L/day). This shows the high water permeability in the kidney versus in other capillary beds. GFR is product of 3 physical factors: 1. Hydraulic permeability of glomerular membrane. If water is allowed through, other dissolved molecules are also allowed through. 2. Total surface area for filtration (c. 2 m2) –LARGE!!! – Product of 1 and 2 is ultrafiltration coefficient Kf (a.k.a. CFC: see Berne & Levy, page 252 3. Capillary ultrafiltration pressure (UP—you pee, I pee, we all pee): high hydrostatic pressure because its upstream from the capillary. This provides the driving force for filtration. B. Ultrafiltration pressure: driving force for glomerular filtration Reflects the balance of forces favoring filtration and absorption— hydrostatic and colloid osmotic pressures. UP is determined by hydrostatic and colloid osmotic pressures in glomerular capillaries, Bowman’s capsule: UP = (PGC + πBC) - (PBC + πGC) In words: UP is the (hydrostatic pressure in the glomerular capillary + colloid osmotic pressure in Bowman’s capsule) – (hydrostatic pressure in Bowman’s capsule + colloid osmotic pressure in the glomerular capillary) Proteins draw fluid towards them—an osmotic effect. Because there’s no protein in Bowman’s Capsule: πBC ~ 0, so UP = PGC - (PBC + πGC) UP is determined by balance of 3 pressures which favor filtration. C. Skeletal muscle capillary At the arteriole end, capillary hydrostatic pressure > capillary colloid osmotic pressure, so there’s net filtration, fluid flows out. Renal #9 Tues, 03/18/03, 11am Dr. Mallet Jennifer Uxer for Jennifer Derby Page 6 of 9 At the venous end, capillary colloid osmotic pressure > capillary hydrostatic pressure, so fluid is net reabsorbed. There is a point where the 2 pressures are equal. Overall in capillary beds other than those in the kidney, the forces favoring filtration balance those opposing it. There’s only slightly more filtration than absorption—about 2 – 4L/day due to lymph flow. D. Glomerular capillary The pressure in the glomerular capillary is very high—about 55mmHg. This is much higher than the pressures in a skeletal muscle capillary. It has a high hydrostatic pressure because it’s upstream from a resistance vessel, the efferent capillary. Glomerular capillaries have a fairly wide diameter and are arranged in parallel, so the overall resistance is low. (Think resistors in parallel: the total resistance is less than any of the individual resistances. 1/Rtot = 1/R1 + 1/R2 + …) As fluid flows from the afferent arteriole end to the efferent arteriolar end, there’s net filtration. This is because of the increase in [proteins] shown by the increase in the colloid osmotic pressure. During filtration, plasma proteins are left behind and are more concentrated as more water is filtered out. There is a net force for the filtration along the length of the capillary. E. Physiological mechanisms for altering GFR GFR = Kf * UP Capillary filtration coefficient: K f = (surface area available for filtration) * (hydraulic permeability). Altered Kf: mesangial cell contraction lowers Kf Altered UP: changes in PGC PGC determined by 3 factors: – Renal arterial blood pressure – Afferent arteriolar resistance – Efferent arteriolar resistance Glomerular mesangial cells can alter Kf loops. Contraction of mesangial cells shortens capillary loops where they are found, lowers Kf and, thus, lowers GFR. When K f is lowered, there’s less surface area for filtration. Angiotensin II promotes the mesangial cells contractraction—this lowers GFR. Other effects of angiotensin II increase GFR. They have a contractile protein and function a lot like smooth muscle. KEY CONCEPT: GFR is physiologically controlled by adjusting resistance of afferent and efferent arterioles Afferent arteriolar constriction: – Greater pressure drop upstream of glomelular capillaries – PGC falls, which lowers GFR Renal #9 Tues, 03/18/03, 11am Dr. Mallet Jennifer Uxer for Jennifer Derby Page 7 of 9 – resistance to flow → ↓ pressure in the capillary → ↓ GFR – Renal blood flow falls ( resistance) Efferent arteriolar constriction: – Downstream from glomerular capillaries – Pooling of blood in glomerular capillaries – Increased PGC increases GFR – Angiotensin II causes this effect. – What would happen to renal blood flow? It decreases because of the increase in resistance. – Here, GFR can increase while renal blood flow decreases. F. Things affecting pressure in the glomelular capillary PGC is affected by 1. systemic arterial pressure (PA)—there’s a change in glomerular hydrostatic pressure and a change in the rate of filtration, 2. afferent arteriolar resistance (RA) — ↓ P, ↓ GFR, ↓ blood flow 3. efferent arteriolar resistance (RE) — P, GFR, ↓ blood flow The pressure in the peritubular capillaries is then lowered. This provides more time for filtration The ‘garden hose’ analogy—think of the afferent arteriole, glomelular capillary, Bowman’s capsule, the efferent arteriole as a garden hose. There’s a hole in this hose—that’s equal to the glomeluar capillary with water filtration into Bowman’s capsule. Scenario 1: Increased systemic arterial pressure water into the hose = systemic arterial pressure. water spraying out of the hole in the hose = GFR due to the systemic arterial pressure. water out of the end of the hose = renal blood flow. Note that auto-regulation minimizes these changes in GFR and renal blood flow due to a change in systemic arterial pressure. Scenario 2: Afferent arteriolar constriction The hose is constricted proximal to the hole in it. So, there’s very little water out of the hole and out of the end of the hose. This is ↓ in GFR and ↓ in renal blood flow. Scenario 3: Moderate efferent arteriolar constriction The hose is constricted distally to the hole. Now, there’s water spraying from the hole = GFR, but there’s ↓ water pouring out of the end of the hose = ↓ renal blood flow. G. Hydrostatic pressures in renal microcirculation: effects of arteriolar constriction See the power points for the graph. Don’t memorize the values. Know changes or ↓. Normal: afferent arterioles present some resistance, so the pressure will fall. The pressure is high in the glomelular capillary beds. There’s a further drop Renal #9 Tues, 03/18/03, 11am Dr. Mallet Jennifer Uxer for Jennifer Derby Page 8 of 9 V. in the efferent capillaries, and the hydrostatic pressure is low in the peritubular capillaries. Afferent constriction: greater pressure ↓ upstream of the glomelular capillaries, ↓ GFR, ↓ peritubular pressure Efferent constriction: pressure because the pressure drop is downstream of the glomelular capillaries, glomelular filtration, ↓ peritubular pressure Autoregulation Autoregulation of renal blood flow and GFR (The graphs shown in the ppts are more accurate than in Fig. 40-18, p. 693) Rates are fairly constant in large pressure changes of mean arterial blood pressure from 80 – 180 mmHg. A. Mechanisms for auto-regulating renal blood flow and GFR 1. Myogenic response to increased systemic arterial pressure Myo = muscle—muscle constrictions primarily regulate renal blood flow. GFR is regulated as a result of this. Systemic arterial pressure stretches blood vessels: cortical radial arteries & afferent arterioles it constricts in response resistance renal blood flow pressure stretch relaxation. Increased vascular resistance renal blood flow PGC The opposite occurs if systemic arterial blood pressure falls. 2. Tubuloglomerular feedback responses to: – Increased GFR – Decreased GFR – This a is communication between the nephron tubule and the glomeluar circulation. This is mediated by the juxtaglomerular (JG) apparatus. B. Autoregulatory mechanisms involving juxtaglomerular apparatus Located at beginning of distal convoluted tubule. Cells of the JG apparatus lie near the glomerulus. Components found at the beginning of the distal convoluted tubule: – Macula densa: in wall of distal convoluted tubule. It is a dense patch of cells that monitor rate of fluid flow through the distal convoluted tubule and signal to the adjacent arterial cells to constrict or relax. – Extraglomerular mesangial cells—mediators between the macula densa and the granular cells, and help transfer the signal. – Juxtaglomerular (granular) cells in afferent & efferent arteriole smooth muscle. Granular cells contain renein. Juxtaglomerular apparatus responds to blood pressure changes to maintain GFR nearly constant. C. TGF response to increased renal blood pressure Initially, there’s an increase in GFR because of the increased hydrostatic pressure in the glomelular capillaries. Renal #9 Tues, 03/18/03, 11am Dr. Mallet Jennifer Uxer for Jennifer Derby Page 9 of 9 More fluid filtered into the nephrons and flows thru. This is sensed by the macula densa. The macula densa signals cells in the adjacent arteriole cells to constrict. The signaling is done using adenosine. There’s now afferent arteriole constriction causing a decrease in flow and a decrease in GFR. See power points for the entire flow chart. D. Response to decreased renal blood pressure GFR falls; less Na+, Cl- filtered and less delivered to macula densa, which senses this drop in fluid delivery Macula densa signals granular cells to secrete renin, which catalyzes the production of angiotensin II. Increased circulating angiotensin II: – Potent vasoconstrictor restores blood pressure – Efferent arteriolar vasoconstriction in the kidney which restores GFR – The afferent arteriole is not as sensitive to angiotensin II, so it doesn’t constrict as much. – Why do we want to continue filtering? To eliminate waste products. – Maintenance of GFR when systemic arterial pressure has fallen it allows removal of waste while the constriction of the efferent arteriole diverts renal blood to the rest of the body and stabilize blood pressure.