ct 102 inorganic chemistry i
advertisement

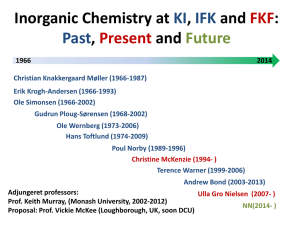
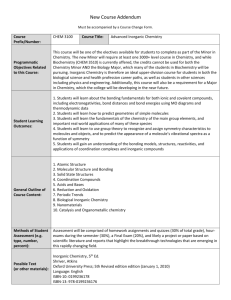
MIDLANDS STATE UNIVERSITY FACULTY OF SCIENCE AND TECHNOLOGY DEPARTMENT OF CHEMICAL TECHNOLOGY CT 102 INORGANIC CHEMISTRY I PREAMBLE The course is a foundation for Chemistry in general upon which the understanding of Chemistry in general is based . It is especially important to have sufficient appreciation of the theories of bonding and how some atomic and molecular properties such as magnetism and electronic properties can be explained from the arrangement and distribution of electrons in a species . Coordination Chemistry introduces the very wide topic of Transition and Further Transition elements and their compounds . The practical component serves to augment these two main sections . COURSE OUTLINE CT102 A: Atomic Structure: -Quantisation of energy and radiation ;Wave Mechanics. -Hydrogen atomic orbital and quantum numbers -Penetration effects. Pauli exclusion principle -Aufbau principle. Hund’s rules. Electronic structure of atoms. -Periodicity of atomic properties -Ionic bonding. Lattice energies -Polyatomic molecules -Hydrogen bonding -Metallic bonding CT102 B: Transition Metal Chemistry: -Introduction to transition metal and coordination chemistry. -Electronic structure and periodicity of transition metal properties -Coordination numbers and structures, types of ligands, nomenclature and isomerism. -Introduction to bonding in coordination compounds in terms of Valence and Crystal field Theories. -Introduction to the magnetic properties of transitional metal complexes. PRACTICAL INORGANIC CHEMISTRY. Experiments on preparation and properties of selected transition metal compounds ASSESSMENT 1 2 3 4 Assessment will be by coursework and written exam Coursework shall constitute 40% of final assessment (10% Tests, 25% Practicals and 5% Assignment ) Written exams shall constitute 60% of final assessment To pass a candidate must score an overall of at least 50% coursework and examination combined READING LIST 1. 2. 3. 4. 5. M. Mahan and Rollie J. Meyers “ University Chemistry” , latest edition preferable ) , 1987, ; Benjamin / Cummings Publishing House Inc . K.M MacKay and R.A Mackay , “ Introduction to Modern Inorganic th Chemistry” , ( 5 edition ) , Blackie Academic and Professional . Cotton , Wilkinson and Gaus , “ Basic Inorganic Chemistry” (latest edition) , John Wiley and Sons Inclusive William L. Jolly, “Morden Inorganic Chemistry (international edition) , 1989 or latest Any textbook on Inorganic Chemistry