carilion medical center institutional review board
advertisement

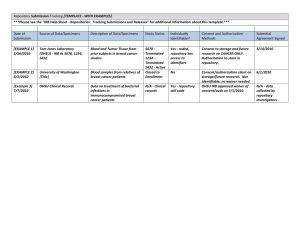
CARILION CLINIC INSTITUTIONAL REVIEW BOARD Standard Operating Guidelines Title: 3.11 Reviews Requiring Special Consideration: EXTERNAL REQUESTS FOR PROTECTED HEALTH INFORMATION FOR RESEARCH USE Original Date: October, 2010 Date of Last Revision: Primary Sponsor: Department of Approved By: Director of Biomedical and Biomedical and Research Ethics Research Ethics, IRB Chair, IRB Vice-Chair Objective: To describe the process to review requests from external groups for protected health information for research use. General Description: From time to time external groups make requests to Carilion departments (e.g. Health Information Management or diagnostic or imaging laboratories) to obtain protected health information for the purpose of conducting human subjects research. In order to assure that these requests meet federal and state regulations governing human subjects research regarding privacy and confidentiality, the Carilion IRB will review certain documents relating to such requests. Protected health information cannot be released to external groups until the Carilion IRB provides written approval to the department to release the information. Procedure: Whenever Health Information Management or any other department receives a request from an external group to obtain protected health information for research purposes, it will send a copy of the correspondence and attachments to the Carilion Clinic IRB, c/o IRB Administrator, Crystal Spring MOB, Suite 202, 2001 Crystal Spring Avenue, Roanoke, 24014. The IRB Administrator and IRB Human Protections Administrator will review the correspondence and make sure the following documents are provided: The Federal Wide Assurance number of the institution conducting or sponsoring the research. A copy of the IRB approval letter for the research project, including waiver of informed consent. A copy of and IRB/Privacy Board waiver of HIPAA authorization. A copy of the research protocol. A copy of the data collection form(s) used in the research. The IRB will inform the department in writing that the information can be released to the external researcher(s) once the above documents/data have been received and the project has been approved by the Institutional Official. The Carilion Clinic IRB considers research that meets the above criteria DOES NOT engage Carilion in human subjects research as outlined in the Office for Human Research Protections 1.2: Page 1 of 2 2008 document entitled “Guidance on Engagement of Institutions in Human Subjects Research.” Specifically, the OHRP guidance documents states in section B. 6 that institutions are not engaged in research when their “employees or agents release to investigators at another institution identifiable private information or identifiable biological specimens pertaining to the subjects of the research. Note that in some cases the institution releasing identifiable private information or identifiable biological specimens may have institutional requirements that would need to be satisfied before the information or specimens may be released, and/or may need to comply with other applicable regulations or laws. In addition, if the identifiable private information or identifiable biological specimens to be released were collected for another research study covered by 45 CFR part 46, then the institution releasing such information or specimens should: (a) ensure that the release would not violate the informed consent provided by the subjects to whom the information or biological specimens pertain (under 45 CFR 46.116), or (b) if informed consent was waived by the IRB, ensure that the release would be consistent with the IRB’s determinations that permitted a waiver of informed consent under 45 CFR 46.116 (c) or (d). Examples of institutions that might release identifiable private information or identifiable biological specimens to investigators at another institution include: (a) schools that release identifiable student test scores; (b) an HHS agency that releases identifiable records about its beneficiaries; and (c) medical centers that release identifiable human biological specimens. Note that, in general, the institutions whose employees or agents obtain the identifiable private information or identifiable biological specimens from the releasing institution would be engaged in human subjects research. [See scenario A.(6) above.] If the research project under review does not meet the criteria for non-engagement in research, then the researchers conducting the study will be advised that they need to submit an application for formal review to the Carilion Clinic IRB. 1.2: Page 2 of 2