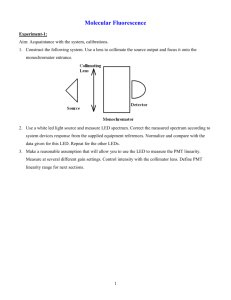
Introduction:
advertisement

Introduction: Fluorescence occurs when a molecule absorbs light photos from the UV light spectrum. The relationship between absorbed and emitted photons at specified wavelengths is the character of Fluorimetry. The intensity of fluorescence relates to the concentration of fluorescent dye. In this experiment, the intensity of fluorescence of fluorescent dye, fluorescein on different pH will be determined. Observation: 1. Beside the NaOH (0.1M) solution, a prepared HCl (0.05M) solution was also used to obtain the pH solutions. 2. Instead of glass quartz cuvette, a plastic quartz cuvette was used in the experiment. 3. NaOH solution was stored in a plastic bottom. Data Table Fluorescein : excitation wavelength: 494nm Emission wavelength: 518nm pH Intensity 6.93 103798 8.01 4164.57 8.47 144979 9.02 247722 9.5 8664.09 10.11 253825 Discussion: Both of the wavelength and the emission intensity are likely to be different for the ionized and nonionized forms of the compounds.(1) Fluorescein is a buffering solution, as a the pH of the solution changed, the reaction direction moved. It affects the concentration of the ionized and nonionized fluorescein representing in the solution. HO O O HO O O O OH O ---------- O - + H+ The pKa value of this buffering equation is 6.4. The intial concentration of Fluorescein 25ml/50ml * 0.2M = 0.1M Sample Calculation: pH= pKa –ln([fluorescein-]/[fluorescein]) At pH= 6.93 6.93= 6.4- ln [(0.1-[fluorescein])/[fluorescein]] [fluorescein-]/[fluorescein]= e-(6.93-6.4) = 0.5886 [fluorescein-] = 0.1-[fluorescein] [fluorescein]= 0.1/(1.5889) = 0.0629M Concentration of fluorescein (M) 0.0629 0.0833 0.0888 0.0932 0.0957 0.0976 pH Intensity (count/sec) 6.93 103798 8.01 4164.57 8.47 144979 9.02 247722 9.5 8664.09 10.11 253825 The power of fluorescence intensity F is proportional to the concentration of the molecule (F=Kc).(1) As the concentration of the molecule increases, the intensity of the fluorescence increases. From the data, it can determine that the molecule fluorescein form is more likely to the fluorescence emission intensity. In this data, the concentration of fluorescein molucule represented in each pH can be read. As the pH value increases, the concentration of the fluorescein molecule existed in this solution increases. Therefore, as the pH increases, the fluorescence intensity should increase as well. However, in the data table, the fluorescence intensities of pH 8.47 and 9.5 solutions did not showed as they should be. This may because the intial concentrations of Fluorescein in the pH 8.47 and 9.5 solution are less than other solutions. It may observed from the color of the solutions. The solutions of pH 8.47 and 9.5 are much brighter than other solutions because less fluorescein presented. In sum, the measured intensity of pH 8.47 and 9.5 solutions can be considered as errors. The result is that as the pH increases, the fluorescent intensity increases. Questions: Q1) plot fluorescent intensities versus pH Because the intensity of pH 8.01 and 9.5 is too small can be considered as an error, the plot should be like the following: Q2) Discuss the shape of the curve and the chemistry involved. Just simple read from the curve, as the pH value increases, the intensity of fluorescent increases. By the calculation of the number of fluorescein molecule in the solution, the fluorescence intensity versus pH shape can be analyzed. Due to there is a buffering reaction occurs between the fluorescein molecule and fluorescein ions in the each pH fluorescein solution. Fluorescein <-----------> Fluorescein- +H+ pKa = 6.4 When pH<6.4, more H+ represents, it helps the reaction move backward, therefore, all of the fluorescein is exist as fluorescein molecule. While pH > 6.4, as the pH increases, the concentration of neutral fluorescein increases. Because of the fluorescence intensity is proportional to the concentration of fluorescein, the fluorescent intensity increased as the pH value increased due to its concentration of fluorescein molecule increased. In the fluorescein intensity and pH plot, the fluorescent increased rapidly from pH 6.93 to 9.02, and then the curve became nearly constant when pH was 9.02 or greater. This is because the concentration of fluorescein molecure relative increases rapidly at the pH range 6.93 to 9.02; the concentration of the neutral fluorescein relative increases relaxedly as the pH value was greater than 9.02. Q3 Describe two other solution conditions that will alter the quantum yield of fluorescein. Except pH effect, both temperature and solvent amount also effect the quantum yield of fluorescein. Effect of Temperature The quantum yield of fluorescein decreases as the temperature increases because the collisions increase at increased temperature that improves the probability for deactivation by external conversion.(1) In sum, high temperature causes low quantum yield of fluorescein; low temperature causes high quantum yield.(2) Effect of Solvent Similar to the effect of temperature, due to the less molecule collisions the better quantum yield, more solvent in the solution will decrease the frequency of molecule collisions. Therefore, the less solvent the worth quantum yield; the more solvent the higher quantum yield.(1) Q4) describe a bioanalytical application that uses flurescein for detection. Fluorescein angiography is a test which allows the blood vessels in the back of the eye to be photographed by use fluorescein for detection.(3) Fluorescein sodium is a fluorecent dye which absorbs blue light with fluorecence.(1) As the fluorescein is injected into the bloodstream, the blood vessels in the back of the eye can be photographed as the fluorescein moves along the blood vessels. It helps the doctor to confirm a diagnosis. Conclusion: pH sometimes affects the intensity of fluorescence. If the fluorescence dye is a weak acid which is a buffering system, the pH will change the intensity of fluorescence. Because of the buffering system will change the concentration of the acid molecule in the solution. In this experiment, fluorescein was used to determine the pH effect on the intensity of fluorescence. Because the fluorescein is a weak acid, the pH changing will affect the concentration of the fluorescein molecule and further affect the intensity of fluorescence. Except pH effect, temperature and solvent also can infect the quantum yield of the molecule. Therefore, in order to control the characteristics of fluorescence, the infecting factor should be considered or try to keep them in constant. References: 1) SKOOG. HOLLER. NIEMAN. Principles of Instrumental Analysis, 5th ed. ; Brookes Cole; United States of America,1997;Vol.6, p 355-365. 2) Introductory Instrumental Analysis: Chemistry 316 Laboratory Manual; Simon Fraser University; Burnaby, 2010; p 13-21. 3) Wendy Strouse Watt, O.D. Fluorescein Angiogram, July, 2002. Molecular Spectroscopy: Effect of pH on quantum yield of fluorescein MiaoMiao Gu 301100545 Chem 316 Monday, Sep. 27,2010