Synthesis of Sulfonamides and Determination of Biological Activity
advertisement

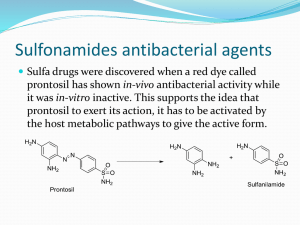
Synthesis of Sulfonamides and Determination of Biological Activity and it Relation to Kow Chem 36B Introduction Sulfonamides are antibiotics commonly used to treat infectious diseases. Their development led to a medical revelation in drug treatments. One of the most important factors that determine this effectiveness is the sulfonamide’s lipophilicity toward the infected cell’s plasma membrane. Lipophilicity is the affinity a molecule feels for the lipid bilayer of the cell’s plasma membrane and the ease with which the molecules crosses this membrane. To determine the magnitude of the lipophilicity, a partition coefficient (Kow) is found. When the partition coefficient has a large value, the sulfonamide feels a strong affinity toward water insoluble molecules like the lipids in a cell’s membrane. This experiment shows a sulfonamide’s partition coefficient and effectiveness can be determined using two different techniques. The first involves performing a ultraviolet/visible spectroscopy and organizing the data into graphs from which a Kow value can be derived. The second technique utilizes E. coli to test the sensitivity of the bacteria toward each synthesized sulfonamide. A greater sensitivity means the sulfonamide is more lipophilic and therefore has a larger partition coefficient value. In this experiment, six different sulfonamides are synthesized and a partition coefficient is determined for each. Their effectiveness is tested on the gram-negative bacteria, E. coli. It is hypothesized that the sulfonamides, which feel a greater lipophilicity for the lipid bilayer of the plasma membrane of the E. coli cells, are more biologically active and therefore have a greater partition coefficient. The synthesized sulfonamides that are more nonpolar in nature have more biological activity and feel a greater lipophilicity for the plasma membrane of the bacteria cells and therefore have a larger partition coefficient. On the other hand, the more polar sulfonamides are less lipophilic for the bacterial cell’s plasma membrane, which causes the sulfonamide to have a lower level of biological activity and a smaller partition coefficient. The goal of this experiment is to devise an experiment to show a relationship between a sulfonamide’s biological activity and its Kow. The starting materials for the synthesis of each sulfonamide will include sulfanilamide and substituted benzaldehydes available in the chemical inventory. If the hypothesis is proven and the goal is met, the synthesized sulfonamides could be further tested and possibly used in the development of new and more effective drugs. Procedure The first portion of this experiment involves synthesizing different sulfonamides. The synthesis utilizes sulfanilamide, 2-nitrobenzaldehyde, 4-nitrobenzaldehyde, and 4-chlorobenzaldehyde as the starting materials. In a 25 mL Erlenmeyer flask, 800 mg (4.646 mmol) of sulfanilamide and 8 mL (0.174 mmol) of 95% alcohol are heated in a sand bath to completely dissolve the sulfanilamide. The substituted benzaldehyde (492 mg, 3.256 mmol of 2nitrobenzaldehyde and 4-nitrobenzaldehyde and 492 mg, 3.499 mmol of 4-chlorobenzaldehyde) is added to the solution along with two drops of acetic acid. The solution is cooled to room temperature and then placed in an ice bath for 15 to 20 minutes. The flasks are then removed from the ice bath and filtered using a Buchner funnel. The filtered crystals are placed on a tared watch glass and left to dry until the next class period. During the next class period, half of the dried crystals are transferred from the watch glass to a 25 mL Erlenmeyer flask and 12 mL (0.26 mmol) of 95% alcohol is added. The solution is heated in a sand bath to completely dissolve the crystals. When all of the crystals have dissolved, 60 mg (1.587 mmol) of sodium borohydride is added to the solution slowly to avoid excessive bubbling. The solution is mixed for 30 minutes and then acetic acid is added to the solution a few drops at a time until the production of hydrogen gas has ceased. Water (50100 mL, 2.78–5.56 mmol) is added to the solution to dilute it. This solution is heated using a sand bath to completely dissolve the precipitate. The solution is cooled to room temperature and then placed into an ice bath for 15 to 20 minutes. The crystals that form are filtered, placed on a tared watch glass and left to dry until the next class period (2). After the reactions were complete, thin-layer chromatography (TLC) and 1H NMR Spectroscopy is used to test the purification of each sulfonamide and confirm the synthesis of the correct compound, with sulfanilamide as the standard. The NMR sample is prepared by dissolving each sulfonamide in deuterated acetone, dimethylsulfoxide, or acetonitrile and then placing the solution in an NMR tube. Before running TLC, a good mobile phase is found by testing sulfanilamide in different combinations of solvents. Once all of the sulfonamides are synthesized, the mobile phase is tested again and modified slightly to obtain the best separation between the different sulfonamides. The TLC plates are spotted with each sulfonamide and then placed into a TLC developing jar. The plates are then observed under a UV light when developing is finished and the spots are marked so the purification of each sulfonamide can be determined. When synthesis and recrystallization is complete, the partition coefficient for each sulfonamide is determined using liquid/liquid extraction and single beam ultraviolet/visible (UV/Vis) spectroscopy. Different concentrations of distilled water and 1-octanol are put into different reaction tubes. To each tube, a small amount of each sulfonamide is added and mixed using a spatula. These mixtures are then combined in a separatory funnel and thoroughly mixed to ensure that the two solutions come in contact with each other (3). Once the separation is complete and the aqueous layer is removed, a sample of the remaining 1-octanol/sulfonamide mixture is run through a single beam UV/Vis spectrophotometer to measure the concentration of the sulfonamide in the 1-octanol. The different max values obtained from the UV/Vis spectra are used to make a concentration plot from which the partition coefficient is derived using the standard curve (Apparatus 1) (4). To further investigate the partition coefficient of each sulfonamide and its lipophilicity, each synthesized sulfonamide is applied to a pre-made nutrient agar streaked with an E.coli culture. The E. coli is streaked onto each petri plate using aseptic techniques and a few drops of each sulfonamide are applied on top of the streak. The cultures are incubated and later observed. From these observations the effectiveness of each sulfonamide is determined. A culture in which there is a clear zone around the area where the sulfonamide is applied means that the bacteria were sensitive toward the sulfonamide, and a larger zone implies that the sulfonamide has a larger partition coefficient. The following apparatus will be used in this experiment. slits plotter light source sample cell detector grating The materials for this experiment will come from the chemical inventory in the stock lenses and room with the exception of the nutrientmirrors broth, loops, and non-virulent E. coli. These materials Apparatus 1. Single Beam Ultraviolet-Visible Spectrophotometer will come from the microbiology department. Chart 1 gives all of the materials used in this experiment as well as how much of each is used and the cost. sulfanilamide Total Amount to be Used (mg or mL) 2400 mg Total Amount to be Used (mmol) 13.94 mmol 2-nitrobenzaldehyde 492 mg 3.256 mmol $4.10 per gram 4-nitrobenzaldehyde 492 mg 3.256 mmol $1.73 per gram 4-chlorobenzaldehyde 492 mg 3.499 mmol $0.33 per gram sodium borohydride 240 mg 6.35 mmol $1.17 per gram 95% ethanol 80 mL 1.74 mmol $0.02 per mL dehydrated nutrient broth powder 4800 mg ? ? agar 12,000 mg ? $0.19 per gram acetic acid approximately 48 drops (2 mL) 0.033 mmol $0.64 per mL 1-octanol 12 mL 0.092 mmol $0.02 per mL water 50-100 mL 2.78-5.56 mmol petri dish 7 N/A $4.90 each E. coli (non-virulent) ? ? ? Compound Cost (per 1 unit) $1.20 per gram Instrumental This experiment utilizes four different experimental techniques to analyze the sulfonamides synthesized. First, thin-layer chromatography (TLC) plates are used to test the purity and polarity of each sulfonamide. This is important to know for two reasons. Impurities could affect the partition coefficient and how well the sulfonamide penetrates the E. coli cell. Knowing the polarity, allows for a generalization to be made about which sulfonamide has a greater partition coefficient. The sulfonamides that elute farther up the TLC plate are nonpolar and the ones that elute smaller distances are polar. These properties of polarity can be used to determine a partition coefficient for each sulfonamide. The second and third techniques involved in determining the partition coefficient for each sulfonamide synthesized are liquid/liquid extraction UV/Vis spectroscopy. Liquid/liquid extraction is used to pull the sulfonamide into either an organic 1-octanol layer or an aqueous water layer. The sulfonamides that are nonpolar in nature separate into the 1-ocatnol layer and the ones that are polar in nature separate into the water layer. Different concentrations of 1octanol and distilled water are used to see how it affected the concentration of the sulfonamide in each layer. Once the separation is complete, each sample is run through the UV/Vis spectrophotometer to determine the concentration of each sulfonamide. This instrument measures the amount of ultraviolet light absorbed by the sulfonamide sample. The absorptions then give spectra with a max values, which are used to make a plot of the concentration. The curve of the plot is then used to determine partition coefficient of the sulfonamide. Knowing the partition coefficient allows for the inference of how well a sulfonamide will penetrate the plasma membrane of a bacteria cell. For the final analysis of the partition coefficient and how it is related to biological activity, the synthesized sulfonamides placed on cultures streaked with E. coli. Because E. coli is a gram-negative bacterium and have a larger lipid bilayer than gram-positive bacteria, it was chosen as the bacteria species for the cultures. This larger lipid bilayer gives the sulfonamides a larger surface to feel an affinity for, which then makes them have a larger partition coefficient. The sulfonamides that are effective in inhibiting E. coli growth show an area in the petri dish where there is not any growth. The ones that are most effective have larger rings where there is not growth meaning they have more biological activity and feel a greater lipophilicity for the plasma membrane. References 1. Prado, M. A. F. J. Chem Educ. 2001, 78, 533-534. 2. Gilow, H. J. Chem. Educ. 1979, 56, 419-420. 3. Willey, J. D.; Abery, G. B.; Manock, J. J.; Skrabal, S. A. J. Chem. Educ. 1999, 76, 16931694. 4. Minard, R.; Oriskovich, T; Williamson, K. L. Lab Guide for Chemistry 36, 36B, 36H 2002, pp.149-163.