Chemical Formulas and Reactions
advertisement

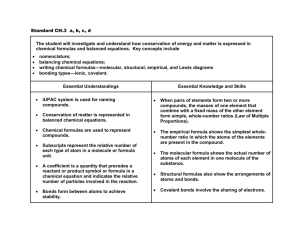
Unit Curriculum Map for Physical Science Cook County Schools Unit: Chemical Formulas and Reactions #6 # of Days: 12 Key Concept(s) Law of Conservation of Matter Classification of reactions Chemical formulas Standards/Elements SPS2. Students will explore the nature of matter, its classifications, and its system for naming types of matter. b. Predict formulas for stable binary ionic compounds based on balance of charges c. Use IUPAC nomenclature for transition between chemical names and chemical formulas of a. Binary ionic compounds b. Binary covalent compounds d. Demonstrate the Law of Conservation of Matter in a chemical reaction e. Apply the Law of Conservation of Matter by balancing the following types of chemical equations: a. Synthesis b. Decomposition c. Single replacement d. Double replacement Unit Essential Questions a. How do the chemicals present in a reaction help you predict the reaction type? b. How can the periodic table help you predict bonding types? c. How do you name and write chemical formulas? d. Why do you need to balance equations? Content Ionic or covalent bonding can be predicted by understanding the properties of the periodic table. There are rules for writing and naming chemical formulas using IUPAC nomenclature. Balancing a chemical equation is essential due to the law of conservation of matter. There are four types of chemical reactions: synthesis, decomposition, single displacement, and double displacement. Key Terms/Vocabulary – “Language of the Standard/Elements” Binary, chemical equation, chemical reaction, covalent bonding, decomposition, double replacement, ionic bonding, IUPAC nomenclature, law of conservation of matter, mass, matter, representative elements, single replacement, synthesis Skills Predict formulas for chemical reactions Identify four types of chemical reactions Balance chemical equations to satisfy the law of conservation of matter Assessment(s) Performance tasks Lab: Types of equations lab. Students complete 4 different lab stations and write balanced equations for the reactions, as well as classifying the type of reaction they observed. They must provide evidence of what they observed. Balancing equation lab: Students will use manipulatives to represent different elements in order to visualize the law of conservation of matter being satisfied. After they have balanced the equations, they need to classify the reaction. Quizzes: Vocabulary, quiz over polyatomic ions Guided practice: flow chart for writing formulas, graphic organizer for ionic/covalent bonds; worksheets on writing formulas, naming compounds, balancing and classifying reactions; worksheets; study guides Test: multiple choice; short answer Self-assessment: graphic organizers; flipbooks for polyatomic ions