Transport phenomena, diffusion
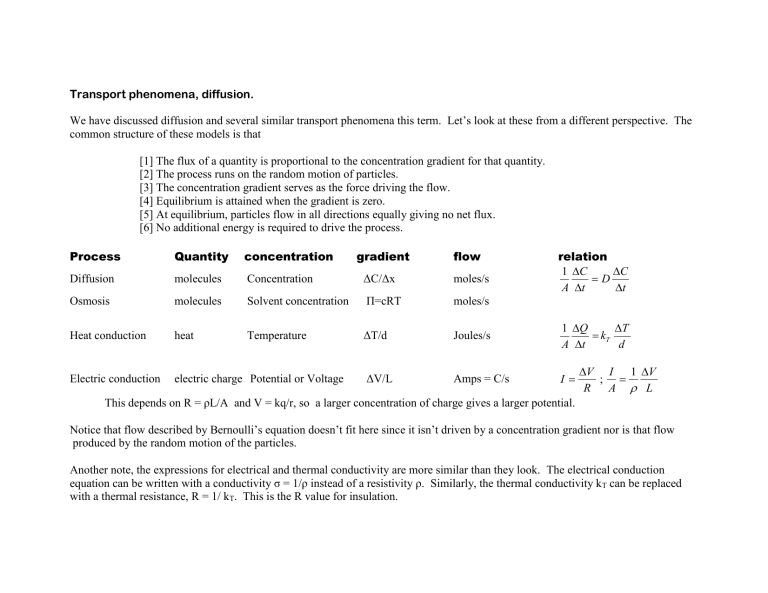
Transport phenomena, diffusion.
We have discussed diffusion and several similar transport phenomena this term. Let’s look at these from a different perspective. The common structure of these models is that
[1] The flux of a quantity is proportional to the concentration gradient for that quantity.
[2] The process runs on the random motion of particles.
[3] The concentration gradient serves as the force driving the flow.
[4] Equilibrium is attained when the gradient is zero.
[5] At equilibrium, particles flow in all directions equally giving no net flux.
Process
[6] No additional energy is required to drive the process.
Quantity concentration gradient flow
Diffusion
Osmosis molecules molecules
Concentration ΔC/Δx
Solvent concentration Π=cRT moles/s moles/s relation
1
C
A
t
D
C
t
Heat conduction heat Temperature ΔT/d Joules/s
1
Q
A
t
k
T
T d
Electric conduction electric charge Potential or Voltage ΔV/L Amps = C/s I
V I
;
R A
1
V
L
This depends on R = ρL/A and V = kq/r, so a larger concentration of charge gives a larger potential.
Notice that flow described by Bernoulli’s equation doesn’t fit here since it isn’t driven by a concentration gradient nor is that flow
produced by the random motion of the particles.
Another note, the expressions for electrical and thermal conductivity are more similar than they look. The electrical conduction equation can be written with a conductivity σ = 1/ρ instead of a resistivity ρ. Similarly, the thermal conductivity k
T
can be replaced with a thermal resistance, R = 1/ k
T
. This is the R value for insulation.