EXPRESSION AND CHARACTERIZATION OF ANTIMICROBIAL
advertisement

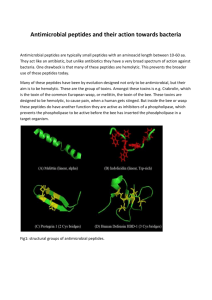
EXPRESSION AND CHARACTERIZATION OF ANTIMICROBIAL PEPTIDE GAMBICIN FROM Culex quinquefasciatus IN Pichia pastoris Phanthila Sirichaiyakul1,*, Rungarun Suthangkornkul1, Apanchanid Thepouyporn1, Okabayashi Tamaki2, Yoshiharu Matsuura3, Naokazu Takeda4, Kazushi Motomura4 Dumrongkiet Arthan1,# 1 Department of Tropical Nutrition and Food Science, Faculty of Tropical Medicine, Mahidol University, Bangkok, 10400 Thailand. *Master of Science in Tropical Medicine (International Program), Thailand 2 Mahidol-Osaka Center for Infectious Diseases (MOCID), Faculty of Tropical Medicine, Mahidol University, Bangkok, 10400 Thailand 3 Department of Molecular Virology, Research institute for Microbial diseases, Osaka University, Osaka, 5645-0871 Japan 4 Thailand-Japan Research Collaboration Center on Emerging and Re-emerging Infections (RCC-ERI), Nonthaburi, 11000 Thailand *e-mail: fairy_alizia@hotmail.com, #e-mail: dumrongkiet.art@mahidol.ac.th Abstract Antimicrobial peptides (AMPs), which can be found in many organisms, are generally low molecular weight peptides containing less than 100 amino acid residues. Due to ineffective conventional antibiotic treatments causing numerous mortalities of infected patients in the world, AMPs are an interesting approach to the development of novel therapeutics against pathogens, particularly antibiotic-resistant bacteria, because they are safe, non-toxic to mammals, no bacterial resistance and showing broad-spectrum antimicrobial activity. While the mosquito AMPs, namely cecropin and defensin have been studied in depth, gambicin has not. Interestingly, an antimicrobial peptide gambicin isolated from Anopheles gambiae cell lines possess antimicrobial activity against gram-positive and negative bacteria, filamentous fungi, and Plasmodium species. The isolation gambicin of Culex genus and expression of recombinant mosquito gambicins remain unreported. This study aimed to express recombinant Culex quinquefasciatus gambicin in Pichia pastoris expression system. We successfully cloned and expressed the Culex quinquefasciatus gambicin in Pichia pastoris. SDS-PAGE revealed obvious two protein bands with a molecular weight of about 7.0 kDa, indicating high production of recombinant Culex quinquefasciatus gambicin. Testing of antimicrobial activity against Escherichia coli by agarwell diffusion assay exhibited no activity. For further studies, a refolding approach to the recovery of proper folding of 3-D structure of the active Culex quinquefasciatus gambicin is required. Additionally, the expression of recombinant Culex quinquefasciatus gambicin in the baculovirus expression system is an alternative method to obtain the active recombinant Culex quinquefasciatus gambicin peptide. Keywords: gambicin, antimicrobial peptides (AMPs), mosquitoes, Pichia pastoris, agar-well diffusion assay Introduction The emergence of antibiotic-resistant pathogens has become a significant global problem, resulting in ineffective conventional antibiotic treatments [1]. Antimicrobial peptides (AMPs) are low molecular weight peptides found in many organisms, which generally consist of less than 100 amino acid residues [2,3]. AMPs exhibit broad-spectrum antimicrobial activity, are safe, non-toxic to mammals, and indicate no bacterial resistance [4,5]. The mechanism underlying AMPs’ action against pathogens is based on the alteration of the biophysical properties of membranes, leading to ion and metabolite leakage from cells, the result of AMPs’ interference with the phospholipids integrity of the cell membrane [6]. Thus, AMPs are of great interest for the development of alternative therapeutic drugs [7]. Presently, AMPs and their derivatives have reportedly been used in many applications including plants and agricultures, food industries, aquaculture, animal husbandry, and drug developments [6,8]. While the mosquito AMPs, cecropin and defensin, have been studied in depth [9,10,11], gambicin has not. Gambicin, isolated from Anopheles gambiae, has been found to possess antimicrobial activity against some gram-positive and -negative bacteria, filamentous fungi, and Plasmodium species [12]. However, the isolation gambicin of Culex genus, recombinant mosquito gambicin production, to test broad-spectrum antimicrobial activity, remains unreported. Since the preferred habitat for mosquitoes Culex genus is polluted water with an abundance of bacteria and microbes [13], it was hypothesized that Culex gambicin may have more effective antimicrobial activity than those from other mosquito gambicins. Thus, it is of great interest in production of Cx. quinquefasciatus gambicin (CQ gambicin) to test broadspectrum antimicrobial activity. This study is aimed to produce recombinant CQ gambicin in Pichia pastoris, characterize its biochemical properties, and test it for antimicrobial activity. Methodology 1. Mosquito samples The fed female Cx. quinquefasciatus were provided by the Department of Medical Entomology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. 2. Primer designs The specific-gene primers were designed from the sequence of Cx. pipiens quinquefasciatus salivary gambicin gene (Accession No. AY388563.1). Forward primer without signal-peptide sequence and reverse primer were included the restriction enzyme XhoI and an XbaI sites, respectively (Table 1). Table 1. The specific-gene primers and applications. Primer names CQ_Gam-XhoI-pZb_F CQ_Gam-XbaI-pZb_R Sequences (5’-3’) GGG GGG CTC GAG AAA AGA GAG GCT GAA GCT TGG GTG TAT GTC TAT GCG AAA ACC TGC GGG GGG TCT AGA TCA CCC AAT GAA GCA CTC GGT AAT GTA ACG Application Amplification of gambicin gene Amplification of gambicin gene 3. Total RNA extraction Female Cx. quinquefasciatus mosquitoes were knocked by freezing at -20oC followed by grounded with liquid nitrogen to obtain the fine powder. To extract total RNA, TRIZOL reagent (Invitrogen, USA) was added. All steps of total RNA extraction were performed according to the instruction manual of Invitrogen (USA). Total RNA extracts were subsequently used as a template for the first-strand cDNA synthesis. 4. Reverse transcription-Polymerase Chain Reaction (RT-PCR) The first-strand cDNA was synthesized according to manufacture protocol (Invitrogen, USA). To synthesize the first-strand cDNA, total RNA extracts about 1 µg was reverse-transcribed by SuperScriptTM III reverse transcriptase cDNA and oligo dT12-18 as a primer. To amplify CQ gambicin gene, the first-strand cDNA was used as a template for PCR reaction containing 1X PCR buffer, dNTPs, Mg2+, specific-gene primers and KOD Hot Start DNA Polymerase (Novagen®). The PCR condition was set as follows: 1 cycle of 95oC for 2 min; 30 cycles of 95oC for 20 sec, 62oC for 30 sec, 70oC for 30 sec; and 1 cycles of 70oC for 3 min. The PCR product was analyzed by 1.7% agarose gel electrophoresis, excised, and gelpurified by Gel/PCR DNA Fragments Extraction Kit (Geneaid, USA). 5. Construction of recombinant expression plasmid harboring gambicin gene The fragment of gambicin gene was cloned into pPICZαB vector at downstream of the α-factor signal peptide sequence. Both of purified CQ gambicin PCR product and pPICZB vector (Invitrogen) were digested with XhoI and an XbaI (TAKARA), and followed by ethanol precipitation, and ligation by T4 DNA ligase (Promega) according to the supplier’s instruction manual (Promaga). Transformation of ligation products into Escherichia coli DH5α, selection of positive clones by LB agar plate containing Zeocin and colony PCR techniques, plasmid extraction and DNA sequencing were carried out to obtain the recombinant pPICZαB plasmid harboring gambicin gene. 6. Transformation the expression plasmid into P. pastoris competent Transformation the expression plasmid into P. pastoris competent was performed according to the instruction manual of Invitrogen. Briefly, not only the recombinant pPICZB plasmid harboring CQ gambicin but also the pPICZB vector were digested with SacI (TAKARA) to make a linear form of DNA, and then transformed into P. pastoris strain GS115 competent cells. Positive transformants were screened from YPDS (1% yeast extract, 2% peptone, 2% dextrose, 1M sorbitol, 2% agar) plates containing Zeocin at a final concentration of 150 μg/ml. 7. Methanol-induced protein expression in recombinant P. pastoris Expression of the recombinant protein in P. pastoris was performed according to the manufacture protocol (Invitrogen). To produce CQ gambicin peptide, both transformant harboring CQ gambicin and pPICZB empty vector were cultured in BMGY (1% yeast extract; 2% peptone; 100 mM potassium phosphate, pH 6.0; 1.34% YNB; 4 x 10–5 % biotin; 1% glycerol) until A600 reading at 5. The cell pellets were harvested and resuspended in BMMY (1% yeast extract; 2% peptone; 100 mM potassium phosphate, pH 6.0; 1.34% YNB; 4 x 10–5 % biotin; 0.5% methanol). Subsequently, the cell solution was further cultured in a shaking incubator at 250 rpm and 16C. Since P. pastoris is methylotrophic yeast, methanol used for induction of expression was added to each cell suspension in BMMY at a final concentration of 0.5% every 24 hrs to maintain induction. After 5 days, the supernatants were harvested by centrifugation and kept at 4 C until used. 8. SDS-PAGE analysis SDS-PAGE was performed with 4% stacking gel and 15% resolving gel according to Laemmli et al [14]. 9. Internal protein sequence analysis The internal protein sequence analysis was performed by in-gel digestion with trypsin and identified by LC-MS/MS at Salaya Central Instrument Facility (SCIF), Mahidol University. The mass spectrometric data were analyzed by mascot MS/MS ions search engine (http://www.matrixscience.com/). 10. Antimicrobial activity assay The antimicrobial activity was determined by agar-well diffusion method. E. coli, a representative of bacteria, was used for antimicrobial test. Briefly, E. coli was cultivated on nutrient agar at 37C for 18 hours, subsequently inoculated in the nutrient broth. It was cultured at 37 C, 250 rpm for 3-5 hours. The cell numbers were adjusted to 0.5 McFarland standards by using 0.9% normal saline solution. These cultured medium was streaked onto Mueller-Hinton agar plate for 3 dimensions and let it dry for 5 minutes. The wells were done by punching with a diameter of 5 mm. All tested solutions and ampicillin as a positive control were filled in agar wells. The agar plate was incubated at 37C for 18-24 hours. The minimum inhibitory concentration (MIC) is defined as the lowest concentration of the recombinant CQ gambicin in agar plates illustrating visible inhibition zone. Results 1. Construction of recombinant expression plasmid The size of the PCR product was about 250 bps, which correspond to mosquito gambicin gene, indicating that CQ gambicin gene was successfully amplified, as shown in Figure 1. The purified PCR product was then constructed into expression vector pPICZB. DNA sequences of two selected positive clones are identical (data not shown). Based on protein alignment, the deduced amino acid sequences of recombinant CQ gambicin shows 100%, 85%, and 73% similarity to those of Cx. pipiens quinquefasciatus (AAR18451.1), An. gambiae (ACA05602.1), and Ae. aegypti (AAL76025.1) gambicin genes, as seen in Figure 2. Figure 1. The PCR product of CQ gambicin gene amplification. Lane 1: DNA marker; Lane 2: CQ gambicin gene; Lane 3: Negative control of the reaction. Figure 2. The alignment of the deduced amino acid sequences of gambicin peptides without signal peptide among CQ gambicin (CQ), Cx. quinquefasciatus (Cx.pq; AAR18451.1), Ae. aegypti (Ae.a; AAAL76025.1) and An. gambiae (An.g; ACA05602.1). 2. Expression and Characterization of recombinant CQ gambicin A clone of P. pastoris expressing the highest recombinant CQ gambicin as judged by SDS-PAGE was cultured in the optimal BMMY, 0.5% methanol expression conditions and compared with a clone of P. pastoris harboring pPICZB as a control. SDS-PGAE analysis of the supernatant of a control (Figure 2; lane 1) and a clone of P. pastoris harboring a CQ gambicin (Figure 2; lane 2) revealed no band and two bands of protein at molecular weight about 7 kDa, respectively. The tryptic digestion of two protein bands on SDS-PAGE was identified by LC-MS/MS. The peptide sequences, observed mass per charge ratios, and molecular weight of the peptides of upper and lower bands were shown in Table 2 and 3, respectively. Ions scores of identical peptides were above 95% confidence. The mass per charge ratios of peptides were subsequently analyzed using Mascot software. These results showed that 5 and 4 tryptic peptide sequences of upper and lower bands were partially identical to deduced amino acids of CQ gambicin. The percents of coverage of matched protein sequence to the upper and lower bands identified by this method were 63 and 52, respectively. Figure 3. SDS-PAGE analysis of supernatants obtained from clones of P. pastoris harboring empty pPICZaB vector (lane1) and CQ gambicin gene (lane2). Table 2. Peptide mass fingerprint of the tryptic-digested recombinant CQ gambicin (upper band) identified by LC-MS/MS. Observed m/z Mr (expt) Mr (calc) Ions score Expect 442.1888 882.3631 882.3654 56 0.41 501.7330 1001.4541 1001.4528 26 5.4e+02 521.7341 1041.4537 1041.4549 62 0.11 606.7866 1211.5586 1211.5605 68 0.027 447.5581 1339.6525 1339.6554 44 6.8 Peptide R.FGTCQDR.Y + Carbamidomethyl (C) R.YITECFIG.- + Carbamidomethyl (C) R.NCGYGSLGSK.K + Carbamidomethyl (C) K.YVSCDGATAIR.N + Carbamidomethyl (C) K.KYVSCDGATAIR.N + Carbamidomethyl (C) Table 3. Peptide mass fingerprint of the tryptic-digested recombinant CQ gambicin (lower band) identified by LC-MS/MS. Observed m/z Mr (expt) Mr (calc) Ions score Expect 442.1889 882.3633 882.3654 53 0.74 522.2256 1042.4366 1041.4549 64 0.083 606.7868 1211.5590 1211.5605 58 0.3 447.5580 1339.6523 1339.6554 22 1e+03 Peptide R.FGTCQDR.Y + Carbamidomethyl (C) R.NCGYGSLGSK.K + Carbamidomethyl (C) K.YVSCDGATAIR.N + Carbamidomethyl (C) K.KYVSCDGATAIR.N + Carbamidomethyl (C) 3. Antimicrobial activity assay The recombinant CQ gambicin peptide was tested for antimicrobial assay against E. coli by agar-well diffusion. As a result, there was no any inhibition zone of the recombinant CQ gambicin in plate comparing to ampicillin as s positive control (Figure 4). Figure 4. The result of agar-well diffusion assay intimating that no inhibition zone (indicating at arrow). (A) recombinant CQ gambicin peptide, (B) pPICZαB (negative control), (C) Ampicillin (positive control), (D) Sterile water (negative control). Discussion and conclusion The cDNA of CQ gambicin was cloned into pPICZαB by fusion gambicin without signal peptide with the Saccharomyces cerevisiae alpha-mating factor propeptide sequence of pPICZαB, and successfully expressed gambicin in P. pastoris culture medium, which is the optimal BMMY, 0.5% methanol expression conditions. SDS-PGAE analysis of the supernatant containing CQ gambicin revealed two bands of protein at molecular weight 7 kDa, corresponding to the size of the mature An. gambiae gambicin as reported previously [12]. This result indicated high production of recombinant proteins. The tryptic digestion of two protein bands on SDS-PAGE was identified by LC-MS/MS. Five and four tryptic peptide sequences of upper and lower bands were identical to the corresponding peptides from deduced amino acids of CQ gambicin. The percents of coverage of matched protein sequence to the upper and lower bands identified by this method were 63% and 52%, respectively. These results obviously indicate that two bands on SDS-PAGE are the recombinant CQ gambicin successfully expressed in P. pastoris. However, two bands of recombinant CQ gambicin found on SDS-PAGE are different from the purified An. gambiae gambicin, which was shown a single band on SDS-PAGE [12]. This result suggested that the two recombinant CQ gambicin peptides may cause by the proteolytic digestion. Additionally, the CQ gambicin exhibited no anti-microbial activity against E. coli, indicating the CQ gambicin is inactive. It may be hypothesized that the inactive CQ gambicin might result from its cleavage by protease. In fact, the An. gambiae gambicin consists of four disulfide bonds [12], which should be similar to CQ gambicin. The improper folding of CQ gambicin caused by the incorrect disulfide bond formation during translation process might affect to its anti-microbial function. To address these problems, further studies required to recover the active CQ gambicin will aim to (i) adding the protease inhibitor such as casamino acid in BMMY culture medium [15], (ii) refolding approach to obtain the correct 3-D protein structure and (iii) expression in the baculovirus-insect system. References 1. Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, et al. The epidemic of antibioticresistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008; 46:155-64. 2. Bulet P, Hetru C, Dimarcq JL, Hoffmann D. Antimicrobial peptides in insects; structure and function. Dev Comp Immunol. 1999; 23:329-44. 3. Bulet P, Stocklin R. Insect antimicrobial peptides: structures, properties and gene regulation. Protein Peptide Lett. 2005; 12:3-11. 4. Lee DG, Park JH, Shin SY, Lee SG, Lee MK, Kim KL, et al. Design of novel analogue peptides with potent fungicidal but low hemolytic activity based on the cecropin A-melittin hybrid structure. Biochem Mol Biol Int. 1997; 43:489-98. 5. Chen GH, Chen WM, Huang GT, Chen YW, Jiang ST. Expression of recombinant antibacterial lactoferricinrelated peptides from Pichia pastoris expression system. J Agr Food Chem. 2009; 57:9509-15. 6. Koczulla AR, Bals R. Antimicrobial peptides: current status and therapeutic potential. Drugs. 2003; 63:389406. 7. Giuliani A, Pirri G, Nicoletto S. Antimicrobial peptides: an overview of a promising class of therapeutics. Cent Eur J Biol. 2007; 2:1-33 8. Keymanesh K, Soltani S, Sardari S. Application of antimicrobial peptides in agriculture and food industry. World J Microbiol Biotechnol. 2009; 25:933-944 9. Maccallum RM, Redmond SN, Christophides GK. An expression map for Anopheles gambiae. BMC genomics. 2011; 12:620. 10. Magalhaes T, Oliveira IF, Melo-Santos MA, Oliveira CM, Lima CA, Ayres CF. Expression of defensin, cecropin, and transferrin in Aedes aegypti (Diptera: Culicidae) infected with Wuchereria bancrofti (Spirurida: Onchocercidae), and the abnormal development of nematodes in the mosquito. Exp Parasitol. 2008; 120:364-71. 11. Harikrishna N, Rao MS, Murty US. Immune peptides modelling of Culex pipiens sp by in silico methods. J Vector Borne Dis. 2012; 49:19-22. 12. Vizioli J, Bulet P, Hoffmann JA, Kafatos FC, Muller HM, Dimopoulos G. Gambicin: a novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae. Proc Natl Acad Sci U S A. 2001; 98:12630-5. 13. Weinstein P, Laird M, Browne G. Exotic and endemic mosquitoes in New Zealand as potential arbovirus vectors [homepage on the Internet]. C2012 [updated 1997; cited 2012 Oct 12]. Available from: http://www.moh.govt.nz/notebook/nbbooks.nsf/0/E3EB410791DF9F974C2565D7000E22AE/$file/mosq2.pdf 14. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227:680-5. 15. Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM. Heterologous protein production using the Pichia pastoris expression system. Yeast. 2005; 22:249-70. Acknowledgements This project was financially supported by the Faculty of Tropical Medicine (Mahidol University). P.S. received the DAAD (Deutscher Akademischer Austausch Dienst) scholarship by the German Academic Exchange Service, and Young Scientist Award 2013 by Merck Millipore Bioscience (Thailand). Additionally, the authors are grateful to Dr. Sungsit Sungvornyothin from the Department of Medical Entomology, Faculty of Tropical Medicine, Mahidol University for providing of mosquito samples. The authors also thank for the assistance of Dr. Amornrat Aroonual, Assist. Prof. Dr. Yuvadee Mahakunkijcharoen and Dr. Onrapak Reamtong.