Fundamental quantities of science
advertisement

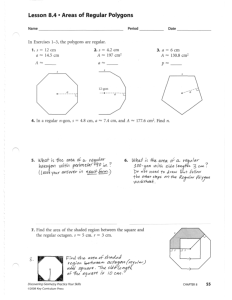
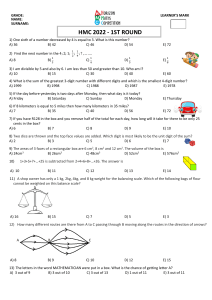
Fundamental quantities of science - SI units Length - meter (m) We'll usually use 'x', 'y', or 'l' to represent distance or length Mass - kilogram (kg) We'll usually use 'm' to represent mass Time - second (s) We'll usually use 't' to represent time The Building Blocks of Matter Molecules Atoms Atoms Particles (Protons, Neutrons, Electrons) Particles Quarks (up, down, strange, charm, bottom, top) Quarks Strings (?) Dimensional Analysis The process of using units (dimensions) to determine relationships between variables. Dimensional analysis cannot determine if there is a "constant of proportionality". Significant Figures Addition/Subtraction: The number of decimal places in the result should equal the smallest number of decimal places of any term in the sum/difference. 4.56 + 2.045 = 6.61 (6.605) Multiplication/Division: The number of significant figures in the final result is the same as the number of significant figures in the least accurate of the values being combined, where least accurate means having the lowest number of significant figures. 3.1 x 6.51 x 0.773 = 15 (15.599913) Conversions Conversion factors are located in the front cover of your textbook. Convert 1200m into ft… 1 m = 3.281 ft 1200 m = 1200 x 3.281 ft 1200 m = 3937 ft Convert 12 cm2 into m2 100 cm = 1 m 1 cm = 1/100 m 12 cm2 = (1/100)2 m2 12 cm2 = 12 (1/100)2 m2 12 cm2 = 0.0012 m2 Convert 4.50 x 103 kg/m3 to g/cm3 Coordinate System We use the Cartesian coordinate system a.k.a. the rectangular coordinate system. Points are labeled (x,y). Sometimes it is more useful to locate a point by its plane polar coordinates (r, ). We use trigonometry to convert from Cartesian (rectangular) to polar coordinates. y = r sin x = r cos r2 = x2 + y2