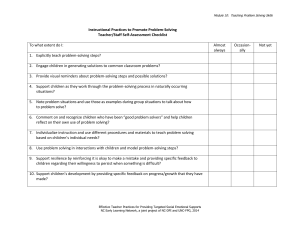
Using Concept Mapping as a Visual Problem
advertisement

STUDENTS’ USE OF PROBLEM-SOLVING TECHNIQUES IN GENERAL COLLEGE CHEMISTRY by Stephen R. Ott A dissertation submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Instructional Psychology and Technology Brigham Young University December 2001 Chapter 1: Introduction Today’s college chemistry students are studying to be the scientists and professionals of the future. To be successful in their chemistry courses, these students must learn how to solve numerous, mathematically-oriented homework problems and test questions. In fact, one of the purposes of these courses is to teach students applicable methods and techniques to solve those homework problems or test questions. These methods and techniques are conventionally called problem-solving strategies by science instructors (Ashmore, Frazer & Casey, 1979; Bodner, 1987; Bunce & Heikkinen, 1986; Chorneyko, Christmas, Cosic, Dibbs, Hamielec, Leod, Moore, Norman, Stankovich, Tyne, Wong & Woods, 1979). Science educators have published lists of successful techniques that good problem solvers use or or characteristics that those problem solvers possess (Bunce, 1984; Herron & Greenbowe, 1986; Larkin, McDermott, Simon & Simon, 1980). Two frequentlyidentified, general techniques from these published lists are categorizing problems and using a general-to-specific process. Using all of these published problem-solving techniques, educators have constructed specific problem-solving strategies to help students be successful in science course. Many of these problem-solving strategies contain a sequential set of procedures that students carry out to solve homework problems or tests questions. Some science instructors have even reported measurable success by students who use these problemsolving strategies (Ashmore, et al., 1979; Bunce & Heikkinen, 1986; Mettes, Pilot, Roossink and Dramers-Pals, 1980; Polya, 1957; Stiff, 1988; see Appendix B). As stated, the vast majority of these problem-solving strategies consist of a set of sequential steps – words, phrases, or other verbal instructions – that students commit to memory and practice while solving science problems. However, psychological research has demonstrated that the majority of students are not verbal learners (Cambell, Cambell & Dickinson, 1992; Fogarty & Bellanca, 1995; Lawrence, 1989; Gardner, 1993; Tobias, 1990, 1992). Therefore, using these strategies would not be as beneficial for non-verbal learners as for verbal learners. To accomodate non-verbal learners, visually-based learning strategies have been demonstrated by science educators (Whitten, Davis & Peck, 2000). One visually-based strategy that has seen increased usage is concept mapping (Regis, Albertazzi & Roletto, 1996; Stensvold & Wilson, 1992). Concept mapping consists of diagramed ideas, connected with lines according to conceptual relationships. (See “Visual Learning Strategies” in this research study.) Their success is attributed to the fact that students are forced to identify and describe relationships between the concepts in a subject area. Using this relationship-based design, concept maps have been identified as a "metacognitive tool" (Regis, et al., 1996), because they help students "learn how to learn" (Novak, 1990). Stensvold and Wilson (1992), found that among students classified with low verbal ability, students who constructed concept maps during the learning process ultimately scored higher on comprehension tests than students who did not. However, although the use of concept mapping a learning strategy has been investigated, research into the use of concept mapping as a problem-solving strategy is lacking. A problem-solving strategy that looks like concept mapping has been in use by science educators for many years (Whitten, Davis & Peck, 2000). These maps contain measurable scientific properties in the map nodes, in place of conceptual ideas that are used in concept maps. The use of these scientific maps will be referred to as property mapping in this research study, in order to emphasize the distinct difference between this type of visual problem-solving approach and concept maps. The purpose of this research was to determine the effect that the use of these verbal and visual problem-solving techniques strategies has on the success of students in a General College Chemistry course. The specific research question that was investigated was “Are students who apply the techniques of problem-solving strategies more successful in general college chemistry than students who do not?” To answer the research question, the following specific, measurable questions were investigated: 1. To what extent do students demonstrate the use of simple problem-solving techniques during examinations? 2. Does the correct use of written problem-solving skills improve students’ performance in examinations? 3. What are the benefits, if any, of using property mapping as a visual problem- solving strategy? 4. Do students’ learning styles (e.g. right-brain or left-brain dominance) influence the effectiveness of different problem-solving techniques? Chapter 2: Literature Review College chemistry students need to develop the ability to solve unfamiliar problems to be successful in the professional world. Instruction into techniques for solving these problems is frequently demonstrated and practiced in college chemistry courses, and the literature describes many strategies taught by science educators. This study investigates the degree to which students’ use of verbal or visual problem-solving techniques increases students’ success in college chemistry classes. This chapter begins by introducing the reader to my personal perception of the need for students to possess intellectual skills to solve unfamiliar problems. The chapter continues by describing how educational theory defines these problem-solving skills, and concludes by introducing the reader to already existing strategies that attempt to develop those skills. Following the introduction, the "Method" chapter contains a description of the experimental design for this study. Following that, the "Results" chapter summarizes the data that was collected and displays various statistical calculations performed on the data. Finally, my conclusions about the research and the effectiveness of the use of problemsolving techniques are described in the "Discussion" chapter. "The Crow and the Pitcher" True problem-solving skills are used not only in educational settings, but all other circumstances of life. I have included the following parable to introduce the reader to the ultimate importance of developing those problem-solving skills. In a spell of dry weather, when the birds could find very little to drink, a thirsty crow found a pitcher with a little water in it. But the pitcher was high and had a narrow neck, and no matter how he tried, the crow could not reach the water. The poor thing felt as if he must die of thirst. Then an idea came to him. Picking up some small pebbles, he dropped them into the pitcher one by one. With each pebble the water rose a little higher until at last it was near enough so he could drink. In a pinch a good use of our wits may help us out. (Scholastic, Inc., 1994) Figure 1. “The Crow and the Pitcher" (Scholastic, Inc.) The circumstances of the crow in Æsop's fable and the impressions of college freshman taking a general chemistry course share a couple of similarities – the environment is hostile and relief is just beyond reach. It is a rare student who does not find chemistry frustrating at one point or another during the semester. Because of the volume and variety of chemical concepts taught in class during the semester, students become overwhelmed tying to learn how to work through all the necessary homework problems correctly. With so much to learn, students wonder if, like the resolution of the crow’s problem, there is a secret technique or "trick" they must discover in order to survive the class. Test questions are particularly frustrating for students. Many feel that although they have studied hard, test questions are unfamiliar or even irrelevant to the subject matter covered in class. Students often label them "trick questions" and consider them unfair. Chemistry instructors counter that there are not trick questions, but that students are expected to learn the necessary concepts and skills in class that provide them with the means to solve test questions correctly. Teachers assume that if students do learn the applicable problem-solving techniques in class, they will be able to answer test questions accurately, and if students do not answer test questions correctly, they did not learn the necessary problem-solving techniques. The purpose of this research study is to determine whether students’ use of identifiable problem-solving techniques does improve their performance in a chemistry class. If students can learn and demonstrate the use of these problem-solving techniques in a chemistry course, those skills should help them to be successful in the professional world. The Problem with Students' Problem Solving As I finish this dissertation, the 21st century is just beginning. As a young boy, I always imagined the 21st century as full of new, almost unbelievable scientific inventions. As an adult, I see the realization of those boyhood imaginings as new scientific ideas, processes, and products arise almost daily. My area of expertise, chemistry, has certainly played a pivotal role in the development of these ideas, processes and products. President Gordon B. Hinckley expressed that same opinion with the following comments that he made at the groundbreaking of the Ezra Taft Benson chemistry building in April of 1993: I am sobered by the thought that during my lifetime there has been more scientific discovery than in all the preceding generations. This is the great age of science. This is the age of chemistry. When I arose this morning, thinking of this occasion, I looked out the window through my plastic lenses—artificial implants in my eye as a result of surgery—and thought, 'Look at the beautiful morning.' . . . I put on clothing that is the result of chemistry. . .. The suit I wear is part wool and part polyester. I put on shoes, the leather of which was tanned through chemistry; the soles of which were made possible through chemistry. I came down here in a car, and as I looked around at the beautiful interior of that car, I noticed all the plastic inside that is the result of chemistry. The beautiful paint on the surface came through the fruits of chemistry. Chemistry has become the very essence of our lives. In fact, when you reflect on it, the greatest of all chemists was the Creator. There will never be another to excel, regardless of what is done in this building or any other building. (Avant, 1993, p. 3) As a chemistry instructor, I am excited to teach the new century's young scientists. Some of these individuals will likely create more of the unbelievable inventions that I imagined as a boy. From my perspective as an instructor, however, one major problem to solve for each of these developing scientists is just that – developing the ability to solve problems. Scientists must develop the skills to solve new problems because in the future, they will not be able to rely solely on already existant scientific processes. The explosion of new information, ideas, and inventions will necessitate synthesizing new procedures to handle new situations. To be ready for this task in their professional careers, it is important for chemistry students to begin developing these problem-solving skills in their college chemistry course. Learning Taxonomies Problem-solving skills exist as part of a larger set of intellectual abilities. Educational psychologists organize these skills into different hierarchies, or learning taxonomies. One taxonomy, developed by Gagné (1992), is listed from lower to higher intellectual skills as Discriminations, Concrete Concepts, Defined Concepts, Rules, Higher-order Rules, and Problem Solving. Gagné's levels are introduced here because they are the most compatible with the taxonomy used by most chemical educators. Chemistry educators usually assume that students in General College Chemistry have developed at least the first four levels of Gagné's intellectual skills, and the literature explains that the purpose of chemistry courses is to develop students' problem-solving skills (Nurrenbern & Pickering, 1987; Pavelich, 1982; Tobias, 1992). However, a clear description of what problem solving consists of is rarely given. The most frequently quoted definition of problem solving in the chemical education literature is expressed (tongue-in-cheek) by Hayes (1981): "Whenever there is a gap between where you are now and where you want to be, and you don't know how to find a way to cross that gap, you have a problem." In practice, chemical educators imply that problem solving is the process of progressing through available facts and processes to arrive at a specific solution. Most of these processes involve algebraic manipulation of mathematical equations, and the exact solution is frequently a numerical quantity. This is the description for problem solving that I will be using throughout this dissertation. Herron categorizes this process as solving a “well-defined” problem, as opposed to an ill-defined problem (Herron, 1996). Techniques of Good Problem Solvers Some science educators have published lists of generalized practices or processes that good problem solvers use. The reader is referred to the three lists by Bunce & Heikkinen (1986), Herron & Greenbowe (1986), and Smith (1992) that are shown in Table 1. These lists contain broad, non-sequential practices used by scientists to solve problems. Other science educators have used these principles to create specific problem-solving algorithms that will be shown later. Common Principles of Good Problem-Solving Techniques Many of the previously-listed practices fit into one of the two following categories of problem-solving techniques that are frequently identified in educational literature: using a general to specific process, or categorizing the problem type. A description follows for each of these categories of practices and their importance in problem solving. Using a General to Specific Process. Researchers have identified that "expert" problem solvers go from a general to specific approach, whereas amateurs work on a more "linear" approach (Larkin, 1981; Reif, 1983). Those researchers stated that experts worked hard at understanding the “whole picture” first, then concentrated on learning specifics. In contrast, novices tried to understand details of problems before understanding where (or if) a specific concept fit into the entire situation. One writer explained it in this way: Table 1. Practices of Expert Problem Solvers Bunce and Heikkinen (1984) 1. Represent the problem verbally. 2. Sketch a diagram of the problem, representing any movement of objects with arrows. 3. Select a set of equations that describe the problem. Herron and Greenbowe (1986) Smith (1992) 1. Work by trial and error. 1. Adapt knowledge and its organization to facilitate the solution of problems in a domain. 2. Think of the problem in terms of the physical system discussed. 3. Solve a special case. 4. Solve a simple problem that seems related to a difficult problem. 5. Break the problem into parts. 6. Substitute numbers for variables. 7. Draw diagrams to represent molecules and atoms. 8. Check interim or final results against other information. 2. Apply knowledge and skills to the problemsolving task. 3. Use forward reasoning and domain-specific procedures on standard problems within the domain of expertise, but use the "weaker" problem solving procedures (means-ends analysis, trial-and-error, etc.) on problems outside the domain of expertise. 4. Create an internal "problem space" which incorporates a qualitative representation or description of the problem. 5. Plan the general strategy or approach to be taken. 6. Break problems into parts and perform multistep procedures. 7. Employ relevant problem-solving heuristics. 8. Evaluate the solution and the solution procedure. 9. Abstract patterns in their own performance and identify useful problem types. This strategy may be illustrated usefully by an analogy to the problem of painting a picture. One painting strategy would be to paint successively, in complete detail, every adjacent square inch of the picture until the total picture is completed. The other strategy consists of first making a rough sketch of the entire picture, then elaborating this sketch by adding more detailed lines, then elaborating further by adding more detailed color information, etc. . . . (Reif, 1983) This general to specific technique can be seen in the procedures listed in Table 1 from ideas such as drawing a diagram or picture, breaking the problem into smaller parts, or restating the problem in other words. One advantage of this process, according to Youmans (1971), is that this helps the students to concentrate more on the process than the final solution. Categorizing the Problem Type. Many scientific instructional practices are based on the work of Piaget (1958), who suggested that information needs to be grouped and classified (Albanese, Brooks, Day, Koehler, Lewis, Marianelli, Rack & TomlinsonKeasey, 1976; Fowler, 1980; Batt, 1980; Bodner, 1986; Brooks, Scholz & Tipton, 1978; Good, Mellon & Kromhout, 1978; Goodstein & Howe, 1978; Johnstone & El-Banna, 1986; Kurland, 1982; Milakofsky & Patterson, 1979; Renner & Lawson, 1973; Wulfsberg, 1983). One application of this problem-solving technique suggests that the student initially should categorize the question into the correct subject area, such as a gaslaw problem, a thermodynamics problem, a stoichiometry problem, etc. (Bunce, Gabel & Samuel, 1991; Chi, Feltovich & Glaser, 1981; Eylon & Reif, 1984; Hinsley, Hayes & Simon, 1977; Mestre, Dufresne, Gerace & Hardiman, 1993; Ryan, 1987). The benefit of this technique is explained by Larkin (1981), who writes that the categorization process is more likely to bring to mind the correct formulas and solution processes. Mestre, et al. (1993), developed software that forced students to categorize a specific homework problem before working it. In their study, physics students were required to categorize problems either according to the mathematical equations that were necessary to solve the problem, or according to the physics concepts that the problem was based upon. The researchers found that students who categorized problems conceptually performed better than those who categorized problems according to the mathematics and equations that were used. Teaching Problem Solving Techniques Teaching students how to use the problem-solving procedures in Error! Reference source not found. has received a great deal of attention in recent years. Books have been written to help students practice solving unfamiliar science problems (Hayes, 1981; Johnson, 1969). An especially comprehensive bibliography covering problem solving in science education can be found in the Handbook of Research on Science Teaching and Learning (Gabel, 1994). Many articles in the literature suggest general instructional practices to encourage the development of problem-solving skills in science students. Two representative lists of these instructional practices are shown in Table 2. Table 2. Instructional Practices to Teach Problem-Solving Skills Gilbert (1980) Halpern (1992) 1. Teach students to read the problem. 1. Set aside laboratory and/or class time for small group problem-solving sessions. 2. Encourage the use of resource materials outside the textbook. 2. Assign problems that require more than the rote application of a previously learned formula. 3. Encourage the use of estimating quantities to solve problems. 3. Teach students to begin the problem-solving task by diagramming the information and/or writing a summary of the given information and the desired answer. Require students to estimate the size of the answer before they begin to solve the problem and check the obtained answer with their estimate. 4. Teach the skill of breaking a problem into parts and solving the sequential parts. 4. While teaching, highlight transferable skills. One frequently-suggested practice, based on the work of Piaget, strongly recommends that subject matter needs to start with concrete concepts that are easily observable in a classroom, and not intangible ideas (Herron, 1975; Gable & Sherwood, 1983). Ryan (1987) adds an additional instructional practice when he suggests that teachers should require that students "name and write down the individual subsets" of the solution process. He claims that students have better success solving homework problems by doing this. Another science educator gives one more important instructional practice by warning that trying to move students up the learning taxonomy too quickly is one of the most harmful teaching techniques (Beistel, 1974). Problem-Solving Strategies By taking the suggestions for good problem-solving practices (Error! Reference source not found.) and the suggestions for good instructional practices (Error! Reference source not found.), specific problem-solving strategies (lists of steps) have been created by science educators as aids to assist students. Instructors hope that by learning and following these problem-solving strategies, students can improve their ability to solve unfamiliar homework problems or test questions. Some of the published problem-solving strategies list very general steps, such as Preparation, Incubation, Inspiration, and Verification (Rubenstein, 1975; Ashmore, et al., 1979; Bodner, 1987; Bodner & Pardue, 1995; Polya, 1957; Wickelgren, 1974; Whimbey & Lochhead, 1982). Other strategies list a more specific set of steps for the sciences. Most of these scientific strategies contain steps that emphasize recognizing available data and useful equations or identifying the correct mathematical processes (Bunce, Baxter, Degennaro, Jackson, Lyman, Olive & Yohe, 1990; Bunce & Gabel, 1991; Bunce & Heikkinen, 1986; Chorneyko, et al., 1979; Genyea, 1983; Gendell, 1987; Johnson, 1969; Krulik & Rudnick, 1984; Mettes, et al., 1980; Stiff, 1988; Youmans, 1971). I found that most of these these strategies contain a set of steps that utilizes a general to specific approach that was identified earlier as being important for good problem solving (Genyea, 1983; Reif, 1983). All of the scientific problem-solving strategies that I found in the literature are listed in Appendix B at the end of this dissertation. Three representative examples of these strategies are shown in Table 3. Table 3. Problem-Solving Strategies Polya (1957) Bunce (1986) Gendella (1987) 1. Understand the Problem 1 Given: Information given in the problem 1. Create a clear picture of the physical situation to which the problem refers and describe for yourself that situation in qualitative terms. (a) Restate the problem (b) Select appropriate notation (c) Make a sketch, a drawing, or table 2. Devise a Plan (a) Look for a pattern (b) Make a simpler problem (c) Make a guess and check it (d) Use appropriate labels 3. Carry out the Plan (a) Check special cases (b) Verify the details of the plan 4. Look Back (a) Generalize (b) Find another method of solution (c) Study the method of solution for future reference 2. Asked For: Information asked for in the problem 3. Recall: Rule, equation or principle that is involved in the problem’s solution. 4. Overall Plan: Simplified schematic diagram of the steps needed to solve the problem. 5. Mathematics: Mathematical ratios including the use of dimensional analysis. where needed. 6. Review: Rereading the original problem and the first four steps. 2. Consider the physical principles or mathematical equations that relate the quantities involved in the problem. 3. Devise a series of calculational steps that will enable you to determine what you want to know from the information that is given and the relationships among the quantities involved. 4. Carry out the appropriate calculations. 5. Verify that the answers are reasonable. One strategy introduced by Bunce (1984), called The Explicit Method of Problem Solving (EMPS) has been shown to have some measure of success helping chemistry students. In a study by Bunce & Heikkinen (1986), the instructor demonstrated in class lectures how to use the EMPS strategy to solve problems, but students did not participate in any activity that required practice using the strategy Although the research indicated no statistical difference between students who used their algorithm and those who did not, the researchers indicated that there was evidence the approach was not used to the extent that they had hoped. Students in the study reported that they had found the problemsolving approach to be too time comsuming. A later reviewer noted that "any attempt to change the way novices attempt problem solving may involve more practice than was provided in this study" (Larkin, 1981). Bunce, et al. (1991), later performed a study using EMPS, but with emphasis on the "Recall" step. That step requires some type of problem categorization, and the results of the research showed that there was a definite improvement on scores by those students who had emphasized the recall step. Gabel and Bunce (1994, p. 318) have explained the pedagogical strength of EMPS with the following statement: The Explicit Method of Problem Solving (EMPS) (Bunce & Heikkinen, 1986) which aims to explicitly teach freshman chemistry students (novices) the problem-solving analysis procedures used by experts is another attempt to implement the research findings of cognitive psychology in the college lecture format. A closer look at the EMPS analysis shows how it achieves this by extending the limited capacity of short-term memory . . . while teaching students to analyze problems in an organized fashion. This analysis helps students encode the pertinent information of the problem, which is a major difference in the problem-solving behavior of experts and novices. . .. Encoding as defined by Sternbert . . . is the identification of each term in a problem and retrieval from long-term memory of the attributes of these terms which may be relevant to the problem’s solution. Reif . . . described the use of encoding as a major component of experts’ solution which is often found lacking in novices’ solutions. An important part of the encoding process is problem categorization. If students cannot correctly categorize a problem, they will not be able to retrieve pertinent information from long-term memory for use in solving it. The next part of EMPS helps students relate the parts of the problem that have been encoded, in a schematic diagram of the solution path. Only after the analysis is complete is the use of mathematics and/or dimensional analysis used to reach a numerical answer. Many chemical educators have expressed support for the instruction of specific strategies as an aid to help students develop problem-solving skills. Smith (1991) reminds educators that although we define differences between working exercises and doing “real” problem solving, research involving problem solving almost always includes the process of performing exercises. Smith argues that performing exercises is problem solving if the process requires the student to make decisions within a flexible algorithm (strategy), or if the process requires the student to decide which of several algorithms to use: "The selection of appropriate algorithms and their modification to accommodate the unique aspects of a problem, however, are often important aspects of problem solving." Other chemical educators are supportive of teaching strategies as a necessary beginning to teaching more rigorous problem solving. Reif states that "students must be taught explicit processes to achieve the performance in problem solving that experts demonstrate automatically." Gabel and Bunce claim that "strategies based on Polya's heuristics . . . appear to facilitate students' ability to solve routine problems even though there is some evidence that students may be doing so using algorithms" (Reif, 1994). Some see these specific strategies not as the problem-solving process itself, but an important component (Frank & Herron, 1987). In contrast to those who favor such strategies, Schrader claims that instructors should not teach pre-conceived step-based strategies specifically, but force students to create their own steps. "The use of algorithms [strategies] is not in itself significant. We should try to teach students so that they not only know how an algorithm is used but also why the algorithm works. It is of greater importance to provide the students with opportunities and challenges to create algorithms, for this will enhance their problem-solving skills" (Schrader, 1987, p. 519). Other educators have expressed similar concerns about excessive use of these specific step-based problem-solving strategies. They claim that problem solving involves more than just using a series of steps to reach a solution (Frank, Baker & Herron, 1987; Schrader, 1987), which is more appropriately identified as “just working exercises.” This relationship between real problem solving and merely doing exercises has led some experts to state that strategies are useless to adequately teach problem solving. Some, including Bodner (1991), have sarcastically suggested an "anarchistic" problem-solving algorithm that includes such steps as "Try something"; "Try something else"; and "See where this gets you." The concern these educators have with algorithms is that they give students an incorrect perception that solving problems proceeds smoothly from question to solution, with no dead-ends or misguided tangents. Some educators disagree with teaching any problem-solving strategies because the solutions to real-life situations are not exact enough to program into a computer. One writer comments that "A problem is a task that requires analysis and reasoning toward a goal (the 'solution'); must be based on an understanding of the domain from which the task is drawn; cannot be solved by recall, recognition, reproduction, or application of an algorithm alone; and is not determined by how difficult or by how perplexing the task is for the intended solver" (Smith, 1991). An additional problem with using specific problem-solving strategies is expressed by several science educators who remind us that just because students learn how to follow a set of steps does not mean that they have a conceptual grasp of the subject matter (Cardulla, 1987; Nurrenbern & Pickering, 1987). Cardulla writes that students should conceptually understand the mathematics involved in each step. He uses the specific example that students understand 30 students/classroom, but they can't immediately grasp that 58.5 grams/mole is the same type of relationship. Similarly, states Cardulla, problems cannot be solved algorithmically, i.e., with little or no understanding of what has been done or why it was correct. Visual Learning Strategies One issue that needs to be pointed out with these previous strategies is that all of them are verbally-based—they require students to memorize a sequential set of words, phrases, or statments as instructions. A visually-based strategy—one based upon spatial position of concepts and processes on a page—would probably be preferable for most students. However, I was not able to find any research on the benefits of using visually-based problem-solving strategies. I did encounter several visually-based learning strategies— strategies that help students organize information through visual placement on a page. The following section will briefly introduce three of those visually-based learning strategies: text structure, Vee diagrams, and concept mapping. Text Structure. This learning strategy teaches students to draw boxes on a sheet of paper and write in the different boxes specific learning concepts from reading material. Armbruster, et. al. (1989) claim "children as young as fifth grade can be taught simple text structures that will help them read and write expository text. With the use of a simple, generalizable frame and a pattern for writing summaries, students learned fairly quickly how to attend to and remember the main ideas from problem-solution passages in their classroom textbooks and how to write summaries about what they had read." The four types of text-structure diagrams identified by Armbruster, et al. (1989), are (a)Problem-Solution text structure, (b) Compare-Contrast text structure, (c) Sequence text structure, and (d) Cause/Effect text structure. An example of the Problem-Solution text structure is shown below in Figure 2. Problem [Something bad; a situation that people would like to change.] Actions Result [What people do to [What happens as a result try to solve the problem.] of the action; the effect or outcome of trying to solve the problem.] Figure 2. Text Structure Image A commonly-used text-structure diagram, the flow map text structure, is similar to the problem-solution text structure strategy, except that concepts are placed in boxes based on the sequence of the concepts in a process, and is not based upon any cognitive relationship between the concepts. Anderson and Demetrius (1993) explain that "the flow map may be used to augment other ways of gathering data . . . that place more emphasis on the hierarchical network dimensions and less on the sequential flow of thought." Vee Maps. Vee maps relate different learning concepts together by emphasizing a focal point, which is placed at the bottom of a large letter "V" as shown in Figure 3. Novak (1984) claims that the use of Vee maps is helpful for "human cognitive learning” because it emphasizes the “key role” that “frameworks play in new learning and problem solving." Figure 3. Vee Map Esiobu and Soyibo (1995) performed a study to determine if vee-mapping "would significantly improve student achievement in ecology and genetics. The data obtained confirm the accomplishment of such a significant improvement." The study showed that students who had been taught and used vee-mapping showed more improvement in test scores by the end of the semester than students who had not been taught vee-mapping. The authors claim that using vee maps forces students to be more active in the learning process, since students construct their own relationship between different concepts. Concept Maps. Concept maps have received a great deal of attention in chemical education in recent years as a learning strategy (Novak, 1990; Regis, et al., 1996; Stensvold & Wilson, 1992). Developed at Cornell University during the 1970s, their construction is "based on the epistemological assumption that concepts and concept relationships (i.e., propositions) are the building blocks of knowledge" (Pendly, Bretz & Novak, 1994, p. 9). Figure 4 demonstrates a simple chemistry concept map that shows the relationship of different types of matter (Whitten, et al., 2000). Figure 4. Concept Map on Types of Matter The success of concept maps as a learning tool is attributed to the same principles that make vee-mapping a beneficial learning strategy: students are active learners when they create relationships between important concepts (Esiobu & Soyibo, 1995). Concept maps are further described as a "metacognitive tool,” because they help students "learn how to learn" by organizing information (Regis, et al., 1996; Novak, 1990). Research by Stensvold and Wilson (1992), found that among high-school students who were classified as having low verbal abilities (that group is identified in the research as approximately half of high school students), those students who constructed concept maps scored higher on a laboratory comprehension test than those students who did not not construct concept maps. As described in the literature, concept maps have been used almost exclusively as a learning strategy, and not as an assessment tool or problem-solving tools. Suggestions have been made of practices that might provide valid and reliable results from using concept maps as an assessment tool (Ruiz-Primo & Shavelson, 1996; Wallace & Mintzes,1990). Property Maps. A common problem-solving strategy used by scientists looks like concept mapping, but the map nodes contain measurable scientific properties in place of the conceptual ideas used in concept mapping. To differentiate it from concept mapping, I will refer to it as property mapping in this dissertation. This name not only appropriately differentiates it from concept mapping, but it emphasizes the type of information contained in the nodes and makes it clear that the relationships between the nodes are mathematical formulas or conversions. Chemistry text books have incorporated property maps for many years. The example in Figure 5 demonstrates how concepts are connected by mathematical formulas, and students can use the necessary mathematics to progress from one calculated property to another (Whitten, et al., 2000). As can be seen in this example of a property map, the nodes contain extensive properties that would have scientific units associated with a numerical value. (For example, “Vol A” would have “L”, or “liters” following a numerical value.) The relationship between two nodes is described as a formula or other type of calculation. (“Vol A” and “Mass A” are related by the density formula.) This property map is used to visually show students how to progress from one extensive property to another through the use of an appropriate calculation. And since this property map contains so many nodes, it demonstrates how to progress through several properties to calculate an extensive property that has no direct relationship to the original property. (In other words, it is possible to go through several steps to obtain “F units A” from “Vol A”, even though there is not a direct calculation between the two.) Other property maps such as the one shown in Figure 5 are included in chemistry textbooks as a tool for students to visually see how proceed through successive calculations to solve homework problems. Some students have become so familiar with these property maps that they have reproduced them on scrath paper as aids to solve test questions. Because the use of these property maps can help students solve unfamiliar test questions by using a visual image, I have identified them as a visual problem-solving technique. Two of the possible broad problem-solving skills that I think students would develop using property mapping have already been identified as techniques that good problem solvers use: using a general-to-specific process and categorizing the problem type. When students use or create a property map to solve a problem, they are first looking at the problem as a whole before performing each of the individual calculations. This helps students to develop the general-to-specific view of problem solving. Also, in order for students to use or create the correct property map, they must identify the data to manipulate and the formulas or calculations to use. This forces students to categorize the problem into a specific subject area, which has been referred to as categorizing the problem type. Figure 5. Problem-Solving Property Map This chapter has progressed from learning theory to visual problem-solving strategies, introducing to the reader previous research conducted on problem solving, and that possible benefits exist for chemistry students to use either verbal or visual problemsolving techniques. The following chapter will explain the method used to determine if those benefits exist. Chapter 3: Method Research Question This dissertation has already introduced and explained several problem-solving strategies that are taught and used in secondary and college chemistry. Some of the techniques that are involved in these strategies have been described or identified. A limited amount of research has been done to determine if use of these problem-solving techniques improves students problem-solving skills. As has already been identified in the introduction of this dissertation, the purpose of this research is to answer the question “Are students who use simple problem-solving techniques more successful in general college chemistry?” The experimental design and method for researching this question is described in this chapter. Subjects The subjects in this study consisted of students registered for General College Chemistry (Chemistry 105) during the Winter Semester of 2001 at Ricks College. Students in these courses are usually freshman majoring in one of the sciences, engineering, or a pre-professional track. They are typically 18-22 years of age with a slightly larger number of males than females enrolled in the classes. At the end of the semester, sufficient data from 216 students was collected to use in statistical calculations. Fifty of these students were chosen randomly to learn property mapping with the assistance of a computer software program specifically written for that purpose. Design The students in General College Chemistry are naturally grouped into class sections, taught by different instructors. During the Winter Semester 2001, three instructors taught a total of five sections. Each class section contains approximately 45 students. This research experiment used a quasi-experimental design. Background information was collected on the subject as variables such as ACT scores, collge GPA, and learning preferences. Intervention factors such as course instructor and students’ use of problemsolving techniques were measured and collected as independent variables. The primary focus of this research study consisted of measuring students’ use of problem-solving techniques on the course final examination, and measuring if the use of those problemsolving techniques has an effect on students’ final exam scores, accounting for the contribution of the other measured dependent variables. To measure the level of students’ use of problem-solving techniques, the scratch paper work from the final exam for all students was gathered and I assigned a score for the level of problem-solving techniques that were demonstrated in the students’ work. Hopefully, different problem-solving techniques would be demonstrated by the students as a result of what they had learned from the instructors during the semester or a result of a students’ practice learning property mapping as a visual problem-solving method. This problem-solving score from the final exam was correlated statistically with several other variables that were collected on the students, particularly with their score on the final exam. (A specific explanation of each of these variables is given later.) Through statistical calculations, this experiment attempted to determine if there is a relationship between the degree to which students use problem-solving techniques and their success on the final exam. ChemMap Software As described in the previous section, a certain number of students were selected to learn property mapping as a problem-solving strategy. The instruction in property mapping consisted of computer software that assists students to create property maps as a tool to solve mathematical chemistry problems. This software program was written almost entirely by myself using the authoring software Asymetrix ToolBook. It is named ChemMap. Development of the ChemMap software involved hundreds of hours on my part to write and to construct the appropriate algorithms to give the students necessary feedback during the construction of property maps. This computer code is included in this dissertation in Appendix K. ChemMap creates property maps by allowing students to create nodes (data containers) conncected by relationships (equations or conversions). Since students themselves create the property maps themselves, in place of only using previouspublished propety maps, ChemMap assists students to develop their own visual problemsolving strategies. Figure 6 shows a simple example with two nodes containing the mass and volume supplied by the homework problem. These nodes are connected to a third node where the value of the density has been calculated and entered. The relationship between the nodes is indicated with a circle on the arrows that contains the number “1”. This number indicates which of the boxes at the bottom of the screen displays the mathematical relationship between the nodes. (In this case, the density formula is shown in box “1” at the bottom of the screen.) Figure 6. Example of ChemMap Solving Density Problem To complete the homework problems, such as that shown inFigure 6, students create the nodes and relationships and enter in the appropriate numerical values and scientific units. By constructing the nodes and relationships for this homework problem, the student is using property mapping as a form of problem solving that is similar to how they are demonstrated in chemistry textbooks such as those shown in Figure 5. An advantage of property mapping is that it enables the student to solve longer, multistep problems such as the one shown in Figure 7. In this homework problem, the student creates the nodes and primarily uses the relationships of the Ideal Gas Law (indicated with the arrows labeled with a circle “4”), a stoichiometric relationship from the chemical reaction shown (indicated with the arrow labeled with a circle “R”), and the calculation of the molar mass (indicated with the arrow labeled with a circle “5”). Because the entire property map shown in Figure 7 can be set up before performing any calculations, the student uses property mapping as a problem-solving strategy to plan how to calculate the solution for the homework problem. Figure 7. Example of ChemMap Solving a Gas-Law Problem To test ChemMap as a property mapping instructional tool, I selected 15 students randomly from each of the five class sections to participate in the study with the computer software, and I also invited any interested students to volunteer to use the program. This selection process took place with about four weeks remaining in the semester. Of those invited or volunteering, 25 total students attended one of four special instructional seminars on using the computer software. Each student was asked to complete and submit approximately 25 homework problems, using the software to create a property map for the problem. I periodically reminded students by e-mail and also by personallydelivered letters to work the homework assignments and to visit with me for any necessary assistance. The results of those efforts will be discussed in a later section of this dissertation. A discussion of the ChemMap software is given in Appendix I. The computer interface for ChemMap is displayed in several images in Appendix J, and the complete computer code for the software is included in Appendix K. Instrumentation Quantitative data were collected throughout the semester to use in the statistical analysis when the experiment was completed. These data will be described in this section of this research study, and a summary list for easy reference is shown in Table 4. All of the measured data described in this section are tabulated in Appendix L. Table 4. Summary of Statistical Variables Variable Name Description Instructor General College Chemistry class instructor (3 instructors) HBDI_LR Herrmann Brain Dominance Instrument (1=left, 2=right) HBDI_CL Herrmann Brain Dominance Instrument (1=cerebral, 2= limbic) ChemistryPretest Chemistry test at start of semester (10 points possible) HomeworkCompleted Chemistry class homework completed (0%-100%) StudyHours Average student weekly study hours (decimal value) ChemMapAssignments ChemMap software assignments completed (0%-100%) ProblemSolvingSurvey Problem-solving survey score (30 points possible) ScratchPaperScore Final exam scratch paper score (60 points possible) ACT ACT score (0-35 possible) FinalExam General College Chemistry final exam score (0%-100%) Instructor. Five sections of General College Chemistry were taught during the Winter Semester 2001 by a total of three instructors. Students were grouped according to instructor to determine if differences developed as a result of instruction, particularly since Instructor 3 was already teaching a simplified problem-solving strategy. Chemistry Pretest. Most students involved in the study completed two short diagnostic tests to evaluate initial intellectual capabilities. The first of these was a chemistry pretest, composed of ten multiple-choice questions taken from the American Chemical Society Test-Item Bank. This tests-item bank contained questions from the American Chemical Society National Examination of previous years. (The American Chemical Society National Examination is described more completely below.) These questions contained chemistry concepts taught through the entire semester. A copy of this diagnostic quiz can be found in Appendix C. Brain Dominance Test. The second student diagnostic was the Herrmann Brain Dominance Instrument (HBDI), a test used to classify students in terms of their typical thinking preferences. Students completed questions on personality characteristics that could be used to analyze and identify their specific learning styles (Herrmann, 1989). Student responses to this quiz were analyzed by software that was created and validated through the BYU Electrical Engineering Department. The analysis of the HBDI generates numerical values that make it possible to assign students as either primarily a right-brain learner or a left-brain learner, as well as either primarily a cerebral-brain learner or a limbic-brain learner. Analysis of the HBDI was only possible with assistance from Dr. Brian Woodfield of the BYU Chemistry Department and Richard Swan from BYU’s Center for Instructional Design. Homework Completed. One question on a survey asked students to identify what percent of the homework problems the student completed during the semester from an available list of percentages ranging from 10% to 100%. Study Hours. Students reported weekly how many hours of study outside of class that they had participated in. These hours were totaled for each student and a weekly average was calculated. ChemMap Assignments. Based upon information I had recorded or from information provided by the student, I assigned each student who used the ChemMap software a score that estimated the percent of the assignments that were completed. Problem-solving Survey. At the conclusion of the semester, all subjects completed a survey about their personal use of problem-solving techniques. The survey questions were created from steps or techniques that were common in many of the problem-solving strategies that I had observed (listed in Appendix B). A specimen copy of this survey is shown in Appendix E. Based upon students’ responses to this survey, I assigned scores to estimate the level of problem-solving techniques students had used during the semester. Final Exam Scratch Paper Score. For the final exam, I provided students with scratch paper to write down all test work. Based upon written work for 10 specific problems on the final exam, I assigned a score that estimated the level of problem-solving techniques that students’ had used during the final exam. (This score was based on the criteria for good problem-solving techniques that were identified in the Problem Solving Survey that students had completed earlier.) ACT Score. Each student’s ACT score were extracted from the college computer. This data was used as an additional estimation of students’ initial intellectual skills. Final Exam Score. Each of the instructors administered a national examination prepared by the American Chemical Society (ACS). The examination consists of approximately 80 multiple-choice questions, prepared by a board of chemical educators and tested for validity. The content of the examination questions require a wide variety of intellectual skills to solve, from simple recall of information to algebraic manipulation of scientific formulas. As described above, the chemistry pretest is a selection of questions from previous ACS national examinations, and a copy of the pretest can be seen in Appendix C. A different national examination is created each year by the American Chemical Society, and past examinations are available for secondary and college chemistry departments. The instructors in this study administered previous years’ examinations as the final for the General College Chemistry course. The percent of correct answers on this exam was used as the dependant variable. Subjective Data Besides the quantitative information described in the last section that was used in a statistical analysis, I collected other information which was important to this study. This section describes the other important information that I gathered. Students’ Computer Literacy. Since some of the students were selected to participate in instruction that involved using computer software, there was concern initially that some students’ lack of computer literacy could interfere with their ability to learn property mapping. To determine whether computer illiteracy would be a significant influence, I sent a letter to the computer science instructors on campus with questions regarding students’ computer skills. A copy of that letter is in Appendix D. Four of seven computer science instructors responded, indicating that virtually all students had a fundamental knowledge of how to work computers, such as booting it up and selecting objects with a mouse click. It was determined that no diagnostic quiz on computer skills would be needed. Survey about ChemMap Software. Of the students who practiced property mapping with the ChemMap computer software, twelve of them completed a survey containing questions regarding the effectiveness of the software. The survey can be found in Appendix F. The results of this survey are discussed in the “Discussion” chapter of this dissertation. Survey about Property mapping. The same students who completed the Survey on the ChemMap software also completed a survey about the usefulness of property mapping. This survey can be found in Appendix G. The results of this survey are discussed in the “Discussion” chapter of this dissertation. Follow-up Survey. While grading students’ scratch paper work from the final exam, I was surprised at the amazingly low amount of written work that most students demonstrated. Since the level of written problem-solving skills was an integral variable in this research, I wondered if the problem-solving score students were being assigned was really an accurate indication of the level of problem-solving techniques students actually used during tests. As a check of students’ scratch-paper score, I created and administered a follow-up questionnaire for students who had participated in the research during the Winter Semester 2001 and who were available during the summer school session. This questionnaire can be seen in Appendix H. From the information obtained from this “Follow-up Survey” I determined that for most students, the work on their final exam scratch paper was a correct representation of students’ normal work. (Students were given a copy of the scratch paper from their final exam while answering the questionnaire.) Pilot Project The concept-mapping software was tested by about 35 students in the General College Chemistry class during the Fall Semester 2000. Students completed and printed many problems using ChemMap and they were asked to submit a list of any specific problems and questions that arose while using the software. About 20 students submitted the extra credit assignments but only two students submitted specific comments. Those comments mostly addressed instances when error messages popped-up during the execution of the software. Before Winter Semester 2001, I went through each of the homework assignments again to eliminate more of the possible bugs in the software. Schedule Significant preparation was done on the ChemMap software and on this prospectus during the 1998-1999 school year, after which I devoted full-time to programming during the summer of 2000. Additional programming was completed during the Fall Semester 2000. The research was performed and data gathered during the Winter Semester 2001 and the analysis performed during the current summer break. A summary of the time schedule is displayed in Table 5. Table 5. Project Schedule Event Dates Software development Summer-Fall 2000 Prospectus approval October 2000 Pilot project December 2000 Experiment implementation March-April 2001 Data collection April 2001 Data interpretation April 2001 Dissertation defense August 2001 Graduation December 2001 Budget The only direct cost associated with this research was the price of the authoring software, $600, which was paid for by Dr. John Lamb. However, over $50,000 was invested by BYU and Ricks College to pay for my salary during the 1998-1999 school year while I was learning and working with Asymetrix ToolBook. Of my personal, unpaid time, three summers were invested writing the computer software and completing the dissertation. Chapter 4: Results This chapter summarizes the results of the data collected as described in Chapter 3. The quantitative data (summarized in Table 4) were analyzed using the student edition of Minitab. The entire printout of the Minitab calculations can be seen in Appendix M. The interpretation of these results will be addressed in the "Discussion" chapter of this dissertation. Extent of Problem-solving Strategies Descriptive statistics were calculated for scores taken from the survey that determined the extent of students’ problem-solving strategies (ProblemSolvingSurvey) and for scores assigned to students’ work on scratch paper for the final exam (ScratchPaperScore). The averages and standard deviations of those two variables are presented in Table 6. Table 6. Averages of Data Measuring Students’ Problem-solving Techniques Averages of Data Measuring Students’ Problem-solving Techniques Maximum Variable Subjects Possible Score Mean Standard Deviation ProblemSolvingSurvey 206 36 20.7 2.9 ScratchPaperScore 207 60 21.5 12.0 To analyze for possible differences in the level of problem-solving techniques between students of each instructor, analysis of variance (ANOVA) was calculated for both ProblemSolvingSurvey and ScratchPaperScore. No statistical difference was seen for ProblemSolvingSurvey, but a significant difference was observed for ScratchPaperScore. The statistical analysis in Table 7 shows average ScratchPaperScore for the students of Instructor3 was higher than the averages for students of the other two instructors. This difference was determined to be significant by observing a graph that showed the means and confidence intervals for each of the means. (See Appendix M.) The F-ratio (20.0) and p-value (<0.001) are an indication of high statistical significance for the ANOVA calculation. Table 7. Averages of ScratchPaperScore Instructor Subjects Mean Standard Deviation 1 80 17.1 11.9 2 38 18.0 9.8 3 89 27.1 10.6 Improving Examination Scores Mean scores were calculated for several of the quantitative data from this research study. Those scores are shown in Table 8 for the following variables: the initial diagnostic quiz taken by students (ChemistryPretest); the average of the percent of homework completed by the students (HomeworkCompleted); and the average of the scores for the course final (FinalExam). Table 8. Important Averages and Standard Deviations Variable Mean ChemistryPretest 3.3 of 10 possible points HomeworkCompleted 55% StudyHours 3.9/week FinalExam 65% Standard Deviation 1.8 28.0 1.9 14.8 To measure the relationship that each of the quantitative variables has with the dependent variable, correlations were calculated of each independent variable with the final exam score. The results of those correlations, including the p-value, are displayed in Table 9. Those variables with the highest correlations are used later to calculate a linear regression model for the final exam score. Table 9. Correlation Values with FinalExam Variable Correlation p-value ChemistryPretest .17 .02 HomeworkCompleted -.07 .30 StudyHours -.20 .00 ProblemSolvingSurvey .18 .01 ScratchPaperScore .47 .00 GPA .44 .00 ACT .41 .00 Since this research study includes three categorical variables, the possible interaction of variables upon the final exam scores were calculated. Using ANOVA, is was determined that there was no statistical difference in the scores of students with a cerebral-brain learning preference versus a limbic-brain learning preference (HBDI_CL), but Error! Reference source not found. shows that there was statistically significant difference between the scores of students with a left-brain learning preference versus students with a right-brain learning preference (HBDI_LR). The F-ratio (4.8) and the pvalue (0.03) for this ANOVA calculation indicate that this difference is statistically significant. This difference in the means for FinalExam due to brain dominance is considered later in the calculation of the regression equation for FinalExam. ANOVA of the final exam score was also calculated for the three instructors, and it was determined that students of Instructor1 had a significantly lower average on the final exam score than students of Instructor2 or Instructor3. The results are shown in Table 11. The F-ratio (12.8) and p-value (<0.001) for this ANOVA calculation are statistically significant. This difference of means for FinalExam is also considered later in the calculation of the regression equation. Table 10. Analysis of Variance between Brain Dominance for FinalExam Brain Dominance Subjects Mean Standard Deviation Left 115 66.1 14.8 Right 64 61.0 15.7 Table 11. Analysis of Variance among Instructors for FinalExam Instructor Subjects Mean Standard Deviation 1 85 59% 14.0 2 39 72 % 14.4 3 92 67 % 14.0 To better understand the possible importance that students’ use of problem-solving techniques had on the final exam score, I decided to calculate whether the interaction between students’ use of problem-solving techniques and students’ brain dominance had any influence on the final exam score. In other words, I wanted to know if left-brain thinkers who applied good problem-solving techniques were more successful on the final exam than right-brain thinkers were. (I also wanted to know the same thing about cerebral-brain thinkers and limbic-brain thinkers.) In order to calculate any possible interaction, two new variables were created for use in a statistical analysis. The first of these variables was the calculated interaction of ScratchPaperScore and HBDI_LR (left/right brain dominance). The second new variable was the calculated interaction of ScratchPaperScore and HBDI_CL (cerebral/limbic brain dominance). When these two new variables were included in the step-wise regression that is described below, it was found that neither of them contributed significantly to the final exam score. Since the influence of neither of these two variables upon FinalExam was statistically significant, it was determined that there was no significant interaction between students’ use of problem-solving techniques and students’ brain dominance. To determine which variables would have the most contribution toward a linear regression model, two different statistical calculations, a best subsets regression and forward stepwise regression, were performed using ten predictor variables. Five variables showed up as being most important for the determination of a linear regression model for FinalExam. Error! Reference source not found. shows the five most important predictors of FinalExam. Also shown is the increased percent of R2 that adding each predictor gives to the regression model (Incremental R2), and the total R2 of the regression model after each predictor is added (Cumulative R2). Table 12. Linear Regression of the Most Important Variables for FinalExam Residual Standard Predictor Incremental R2 Cumulative R2 ScratchPaperScore 26.8 26.8 13.3 0.00 ACT 10.1 36.9 12.4 0.00 Instructor 6.4 43.3 11.8 0.00 GPA 7.6 50.9 11.0 0.00 HBDI_LR 1.8 52.7 10.8 0.02 Deviation p Including only these five predictors, a final multiple linear regression equation was calculated. The regression model equation gave the following model for the calculation of the final exam score: FinalExam= - 3.57 + 0.356*ScratchPaperScore + 1.14*ACT + 6.27*Instructor + 10.6*GPA -2.20*HBDI_LR. These five variables in the linear regression model gave an R2 equal to 53.1%. The large F-ratio (32.0) and small p-value (<0.001) indicate the statistical significance of the complete regression model equation. Benefit of Property Mapping Twelve students completed the ChemMap assignments to any degree. Due to lack of sufficient data, no significant calculations can be performed on the benefits of property mapping as a problem-solving strategy on chemistry examinations. Students’ comments from the survey about the use of property mapping are given in the “Discussion “ chapter of this dissertation. Learning Styles and Problem-solving Strategies Of the students who completed the HBDI survey, 115 of the students were classified as left-brain dominant and 64 were classified as right-brain dominant. As already stated above, there was a statistically significant difference between left-brain dominant and right-brain dominant, and that difference was considered in the calculation of the linear regression model for FinalExam. Of the students who completed the HBDI survey, 60 were classified as cerebral-brain dominant and 119 were classified as limbic-brain dominant. There was no statistical difference determined in FinalExam between those two categories. To calculate if one or more specific brain dominance groups would benefit from the use of problem-solving techniques, the interaction effect upon FinalExam was calculated for ScratchPaperScore and HBDI_LR and for ScratchPaperScore and HBDI_CL. ANOVA revealed that there are no significant interaction effects of ScratchPaperScore with any of the learning preference groups. Chapter 5: Discussion and Conclusions The original question asked by this study was “Are students who apply the techniques of problem-solving strategies more successful in general college chemistry than students who do not?” To investigate the answer to that research question, I proposed the following specific questions as the focus of this research study: 1. To what extent do students demonstrate the use of simple problem-solving techniques during examinations? 2. Does the correct use of written problem-solving skills improve students’ performance in examinations? 3. What are the benefits, if any, of using property mapping as a visual problemsolving strategy? 4. Do students learning styles (e.g. right-brain or left-brain dominance) influence the effectiveness of different problem-solving strategies? Each of these questions will be addressed in this chapter in the sections that follow, referring back to the data analyses that are included in the “Results” chapter of this dissertation. Extent of Problem-solving Strategies The amount of problem-solving techniques that students use during tests can be seen from Table 6. The first row in this table, ProblemSolvingSurvey, is an indication of the extent of problem-solving techniques that students indicated they felt they were using. As can be seen, the average score of 20.7 is just over half of the total possible score of 36. This means that students realize that they are not consistently using very many of the problem-solving techniques that were identified in the survey. The small standard deviation for this score is indicative of the vast majority of the students are using very minimal problem-solving techniques, or in other words, that there are very few students, if any, that are consistently using the problem-solving techniques that were identified in the survey. The actual level of problem solving that students used during the final exam is best seen from the second line of Table 6, ScratchPaperScore, which gives an average of 21.5 out of 60 possible points. The variable ScratchPaperScore is a measure of the points that I awarded students from the work they had written down on scratch paper. When I made this evaluation, I gave students points for evidence of each of the problem-solving techniques that had already been identified on the problem solving survey. As a result, ScratchPaperScore is probably inflated. In fact, the single most disturbing observation of this entire research study was the small amount of work that students actually write down while working test problems. I saw numerous cases where virtually nothing was written down for some fairly complicated problems. I can only assume that students did everything in their heads with the assistance of a calculator. This can be a serious problem when students should be keeping track of scientific units and when students should be making certain that their final answer is actually what the question is asking for. This lack of evidence for problem-solving because students students fail to write down important information has been noted by previous researchers during experiments to evaluate the effectiveness of specific problem-solving skills (Bunce & Heikkinen, 1986). Because of this tendency toward minimal writing by students, future research should bear in mind that it will be very difficult to test the effectiveness of specific problem-solving strategies without incorporating a means of accurately measuring students’ use of the strategy. It can be seen from Table 7 that students of Instructor3 received significantly higher scratch paper scores than students of the other two instructors. The difference is likely due to the teaching and grading methods of the instructor. Instructor 3 is one of the most experienced teachers in the Chemistry Department, and I have personally observed that he uses a simple problem-solving technique in his instruction. Of particular interest is that he begins each problem by writing down what the units are for the solution with a question mark (such as ?g or ?g ). This problem-solving technique was observed on ?mol the final exam scratch paper of several students from those sections. (See “Solving Unit Factor Problems” in Appendix B.) I also found out that this instructor requires students to show their work on test questions or no credit will be awarded for answers. This means that students from Instructor 3 were practicing writing down their work during the entire semester. Also of note is that even though all students indicated equally that they used problem-solving techniques (the average of ProblemSolvingSurvey was the same for each of the instructors), only those students of Instructor3 received statistically higher scores on ScratchPaperScore. I believe that much of the success of this “final answer units” method mentioned in the previous paragraph comes from its emphasis on looking at the overall problem before attempting the specific steps. This is the general-to-specific process that others have already identified as being important (Larkin, 1981; Reif, 1983). This method gives students a goal to work towards, in place of some type of “hit-and-miss” method of using various equations. This goal-orientation likely helps students to select the equations or procedures applicable to the correct subject area (Ryan, 1987; Larkin, 1981; Bunce, et al., 1991), and to concentrate on the process of arriving at the correct answer (Youmans, 1971). Improving Examination Scores Table 9 shows the correlation that exists between the final exam and the chemistry diagnostic test, but this correlation is low and doesn’t seem to be very important. There is a statistically significant but low correlation with the HomeworkCompleted and StudyHours variables. The three important correlations for FinalExam are with ScratchPaperScore, GPA and ACT. Table 12 showed that by using a model that included five variables from this research study, 52.7% of the variation in FinalExam can be explained. The relative importance of each of these variables can be seen both from the order that the predictor is added to the regression model and from the increase in the R2 value that each gives to the regression model. Clearly, the amount of work that students’ demonstrate on the final exam (ScratchPaperScore) is the single most important variable, since it is the first predictor added to the regression model and also since it gives the greatest single increase in the R2 for the regression model. The conclusion from the results of the regression model is that students’ written work on the final exam is very important as a technique to increase the score on the final exam. Although previous researchers have indicated that there was evidence for this conclusion, results of previous studies were not as statistically conclusive (Bunce & Heikkinen, 1986). Another conclusion from the results of the regression model shows that long-term, or cumulative, factors are also very important for predicting the final exam. This means that the ACT score and GPA, which are a measure of a cumulation of months or years of intellectual development, have an influence on the success of students in a General College Chemistry course. This is the came conclusion I came to in an earlier study (Ott, 1997). Benefit of Property Mapping Because no statistical calculations could be completed on the use of property mapping, no conclusions can be made about the effect of using property mapping during examinations. However, because several students completed surveys on the use of the ChemMap software and on the use of property mapping, some insight can be shared about the students’ feelings of the potential of both ChemMap and property mapping. The survey that students completed about the use of the ChemMap software can be found in Appendix F. The primary purpose of evaluating the software was to determine if it instructed students sufficiently on the use of property mapping. Only two of the twelve students who completed the survey rated ChemMap as not being user friendly. All but two of the students felt like the software program taught how to use property mapping and they appreciated how the program gave them feedback when they wanted specific calculations checked. Students made comments on the same survey sheet about the use of property mapping to solve test problems. (See Appendix G.) Nine of the twelve (the nine who completed the most ChemMap assignments) felt that property mapping was a better method of solving problems, but only six of them felt that it was an easier method of solving problems. (In actuality, only one of the students wrote down a property map on the final exam.) Students felt that property mapping required a substantial amount of additional writing, which they weren’t willing to do. (They said that it was great to do it on a computer program since property maps could be constructed by dragging data or objects.) Students also indicated that property mapping didn’t contribute to arriving at the correct units for the answer. Several of the students commented to me personally that they visualized property maps in their head for some test questions, even if they didn’t write anything down on paper, and that visualizing the problem in this way was beneficial. Property mapping looked promising as an aid to help students answer test problems correctly, and students did mention that practicing it did help them visualize the entire problem better. However, it had two major deficiencies. First of all, it required more writing than students were willing to do. The benefit received to amount of work done was not seen as profitable. Others have already noted that measuring the success of problem-solving techniques is difficult because students resist using them (Bunce, 1984). Secondly, property mapping did not assist students to cancel units correctly. If it is used, students must still write out all of the formulas and unit cancellations. Learning Styles and Problem-solving Strategies The results of the regression model in Error! Reference source not found. indicate that students with a left-brain preference for learning receive statistically higher scores on the final exam than students with a right-brain preference for learning. This left-brain preference is traditionally associated with logical and mathematical skills, so these results are not surprising (Herrmann, 1989; Swan, 2001) Statistical calculations for the interaction of brain dominance and entent of written problem-solving skills on the final exam showed no statistical variation. This means that the use of problem-solving skills by students of any specific learning style is not more beneficial or less beneficial than by students of another learning style, e.g., left-brain thinkers who use problem-solving techniques don’t statistically receive higher exam scores than right-brain thinkers who use problem-solving techniques. This means that the advantage of writing down the work on a final exam benefits students of all learning styles. Summary Looking at the discussion for each of the four research questions that have just been presented, I will summarize three general findings from this research study, and I will make a few suggestions about possible solutions to problems that this research study has presented. 1. Using problem-solving techniques seems to help students perform better on applicable test questions. But students resist using any techniques or procedures that require more than a minimum amount of writing. Students should be taught to focus on two or three specific problem-solving techniques. Previous researchers (Bunce & Heikkinen, 1986) have already shown that students’ problem-solving skills are more easily measured if instructors simplify the number of techniques that are emphasized. I recommend that further research be performed on the benefit to students of identifying the units for the final answer as the first step. I am surprised that only one of the problem-solving algorithms in Appendix B has this step listed first (Smith, 2001). This technique helps to emphasize the general-to-specific process that has been identified as important. 2. Teachers should give homework that requires more practice with problem-solving techniques and teachers should grade test questions by giving more weight to the adequacy of problem-solving techniques and with less concern on the accuracy of the final answer. From the beginning of the semester, teachers should require that students demonstrate, in writing, the application of specific techniques to receive credit for answers on examinations. Also, I find it interesting that all previous studies on the success of specific problemsolving algorithms mentioned the same problem that I encountered in this research study, i.e., that students do not write down very much during examinations. That means that, like this research study, the full extent of the results are brought into question because of the lack of the evidence that the study is looking for. In other words, I doubt if it is possible to measure the success of any problem-solving technique or problem-solving algorithm without first creating some means to cause students to use the technique or algorithm. Evidently, chemistry teachers are demonstrating problem-solving techniques in class to varying degrees, but they see them as only a means of arriving at the correct answer, and not necessarily as a desirable thinking (problem-solving) method. As a result, examination questions primarily test whether students get the correct answer, assuming that students are using legitimate problem-solving methods when they arrive at the correct answer. The problem with the assumption that students who arrive at the correct answer are using correct problem-solving techniques is that there is not evidence for that assumption. It brings into question whether students really are using problem-solving skills or whether students are only recalling formulas and conversions from similar problems that they’ve already worked before. Is it possible that teachers could design test questions to test problem-solving techniques? Is it possible to create test questions that have no correct numerical answer, but an “algebraic answer” that requires students to work for solutions on paper in place of a calculator. For example, variables could be used in some problems in place of numerical values such as in the following example. Example of Problem-solving Test Question What is the molar mass of a gas if x grams of the gas occupies a volume of y liters at 700 torr pressure and 27 C? (a) 0.0031 xR g y mol (b) 2.33xR g y mol (c) 235.7 xR g y mol (d) 235.7 yR g x mol 3. Property mapping may be helpful as a visual stragegy for students on homework problems if it is available through programmed software. Software would make it possible to create property maps through easily clicking and dragging the necessary components into place. Even if students didn’t use property mapping on tests, practicing to make property maps seems to be beneficial to some students because they can visualize in their mind how to get to the solution of the problem. Conclusions This research study has investigated whether the level of problem-solving techniques that students use on a General College Chemistry examination has an effect on the student’s final scores on the examination. This study confirmed that there is a positive relationship between using problem-solving techniques and the final exam score. This dissertation discussed the evidence for that conclusion and as a result of the evidence, offered suggestions for methods to improve students’s problem-solving skills.