Transformation Protocol_four
advertisement

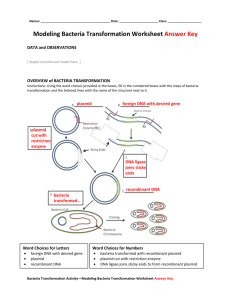
Transformation Protocol 1 Procedure – Transformation of E. coli Cells Your lab station will need: • 2 microcentrifuge tubes • marker • 550 µl CaCl2 on ice • 6 sterile loops • agar slant with E. coli (can be shared between stations) • 15 µl pVib plasmid on ice • 2 “LB/Amp” plates • 2 “LB” plates • 500 µl Luria broth • micropipettes • pipet tips 1. Label tubes a. Label one closed microcentrifuge tube “+ plasmid.” b. Label the other tube “- plasmid.” c. Label both tubes with your initials and place in foam test tube rack. 2. Add CaCl2 a. Using a micropipette with a sterile tip, transfer 250 µl CaCl2 into each tube. b. Place the tubes in the foam rack on ice. 3. Collect E. coli a. Unwrap a sterile loop. b. Use it to collect some E. coli bacteria from the agar slant by gently scraping the surface of the agar. c. Immerse the loop with E. coli into the CaCl2 at the bottom of the “+ plasmid” tube. 4. Suspend E. coli a. Rotate the loop vigorously to dislodge the bacteria. b. Mix the solution by pipetting in and out until there are no floating chunks and solution is cloudy. c. Return the “+ plasmid” tube to ice. d. Use a new sterile loop and repeat steps 3 and 4 for the “- plasmid” tube. Copyright © 2007 MassBioEd Transformation Protocol 2 5. Add plasmid a. Use a micropipette to add 10 µl of pVib plasmid to the “+ plasmid” tube. b. Close the tube and return it to the rack on ice. c. Do NOT add plasmid to the “- plasmid” tube! d. Keep both tubes on ice for 15 more minutes. 6. Label plates While the tubes are sitting on ice, label your four agar plates on the bottom (not the lid) as follows: date/initials + plasmid LB/Amp date/initials + plasmid LB date/initials - plasmid LB/Amp date/initials - plasmid LB 7. Heat shock the bacteria a. Bring your ice bucket with the “+ plasmid” and “- plasmid” tubes to a 42ºC water bath. b. At the end of the 15 minute incubation on ice, transfer both tubes into the water bath for exactly 90 seconds. Use a floating test tube rack to hold the tubes in the water. Make sure to push the tubes all the way down in the rack so their bottoms make contact with the warm water. c. After 90 second incubation, place both tubes back on ice for 2 minutes. d. For best results, the change from the ice to the water bath and back to the ice must be rapid. 8. Add LB broth a. After 2 minutes, remove the tubes from ice and place them in a test tube rack. b. Use a micropipette with a sterile tip to add 200µl of LB broth to the “+ plasmid” tube and close it. c. Use a fresh tip to add 200µl of LB broth to the “- plasmid” tube and close it. d. Keep the tubes at room temperature for 10 minutes. e. After 10 minutes, mix the contents of each tube by tapping with your finger. Copyright © 2007 MassBioEd Transformation Protocol 3 9. Transfer bacteria Using a micropipette and a fresh tip each time, transfer bacteria from tubes to plates. Lift the lid of the plate just enough to admit the pipet tip. Do not allow the tip to touch any surface. a. Transfer 100µl of bacteria from the “+ plasmid” tube to the “LB/Amp + plasmid” plate. b. Transfer 100µl of bacteria from the “+ plasmid” tube to the “LB + plasmid” plate. c. Transfer 100µl of bacteria from the “- plasmid” tube to the “LB/Amp - plasmid” plate. d. Transfer 100µl of bacteria from the “- plasmid” tube to the “LB - plasmid” plate. 10. Streak the plates a. Use a new sterile loop to spread the bacterial suspensions evenly around the surface of the agar. Lift the lid just enough to admit the loop. Drag the flat surface of the loop gently back and forth across the agar surface. b. Use a new sterile loop for each plate. 11. Allow bacteria to grow a. Tape your four plates together. b. Place plates upside down for 36-60 hours at room temperature. c. If an incubator is available, you can incubate the plates at 30ºC for 24-36 hours. However, the bacteria will not glow if incubated at 37ºC. Copyright © 2007 MassBioEd