doc
advertisement
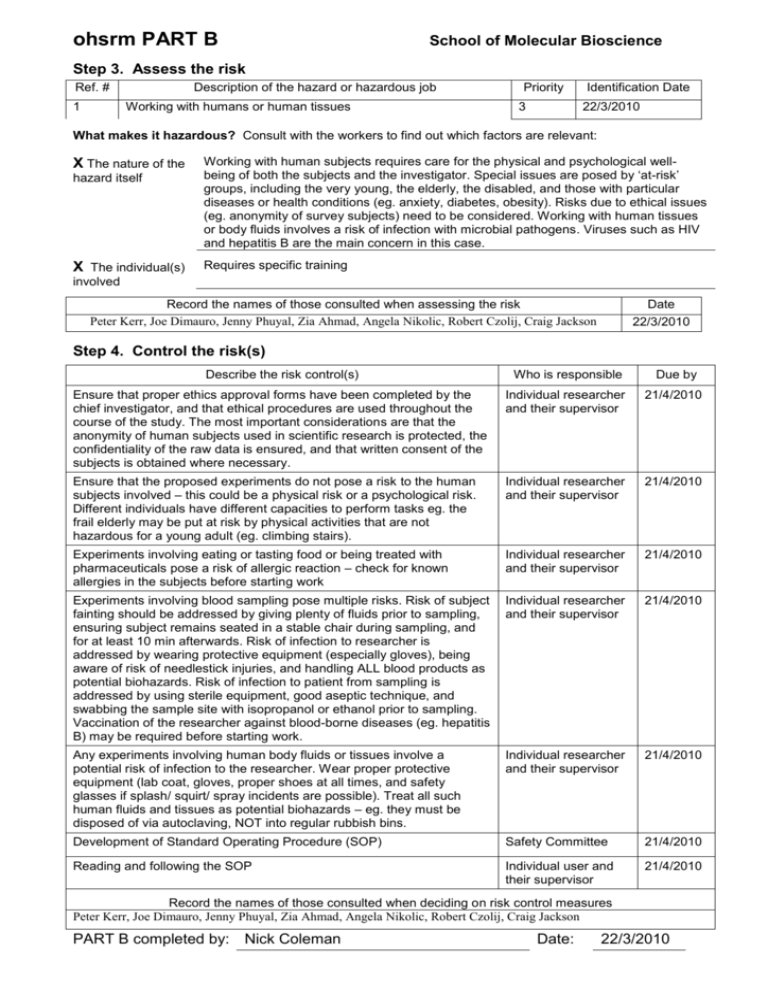
ohsrm PART B School of Molecular Bioscience Step 3. Assess the risk Ref. # 1 Description of the hazard or hazardous job Working with humans or human tissues Priority 3 Identification Date 22/3/2010 What makes it hazardous? Consult with the workers to find out which factors are relevant: X The nature of the hazard itself X The individual(s) Working with human subjects requires care for the physical and psychological wellbeing of both the subjects and the investigator. Special issues are posed by ‘at-risk’ groups, including the very young, the elderly, the disabled, and those with particular diseases or health conditions (eg. anxiety, diabetes, obesity). Risks due to ethical issues (eg. anonymity of survey subjects) need to be considered. Working with human tissues or body fluids involves a risk of infection with microbial pathogens. Viruses such as HIV and hepatitis B are the main concern in this case. Requires specific training involved Record the names of those consulted when assessing the risk Peter Kerr, Joe Dimauro, Jenny Phuyal, Zia Ahmad, Angela Nikolic, Robert Czolij, Craig Jackson Date 22/3/2010 Step 4. Control the risk(s) Describe the risk control(s) Who is responsible Due by Ensure that proper ethics approval forms have been completed by the chief investigator, and that ethical procedures are used throughout the course of the study. The most important considerations are that the anonymity of human subjects used in scientific research is protected, the confidentiality of the raw data is ensured, and that written consent of the subjects is obtained where necessary. Individual researcher and their supervisor 21/4/2010 Ensure that the proposed experiments do not pose a risk to the human subjects involved – this could be a physical risk or a psychological risk. Different individuals have different capacities to perform tasks eg. the frail elderly may be put at risk by physical activities that are not hazardous for a young adult (eg. climbing stairs). Individual researcher and their supervisor 21/4/2010 Experiments involving eating or tasting food or being treated with pharmaceuticals pose a risk of allergic reaction – check for known allergies in the subjects before starting work Individual researcher and their supervisor 21/4/2010 Experiments involving blood sampling pose multiple risks. Risk of subject fainting should be addressed by giving plenty of fluids prior to sampling, ensuring subject remains seated in a stable chair during sampling, and for at least 10 min afterwards. Risk of infection to researcher is addressed by wearing protective equipment (especially gloves), being aware of risk of needlestick injuries, and handling ALL blood products as potential biohazards. Risk of infection to patient from sampling is addressed by using sterile equipment, good aseptic technique, and swabbing the sample site with isopropanol or ethanol prior to sampling. Vaccination of the researcher against blood-borne diseases (eg. hepatitis B) may be required before starting work. Individual researcher and their supervisor 21/4/2010 Any experiments involving human body fluids or tissues involve a potential risk of infection to the researcher. Wear proper protective equipment (lab coat, gloves, proper shoes at all times, and safety glasses if splash/ squirt/ spray incidents are possible). Treat all such human fluids and tissues as potential biohazards – eg. they must be disposed of via autoclaving, NOT into regular rubbish bins. Individual researcher and their supervisor 21/4/2010 Development of Standard Operating Procedure (SOP) Safety Committee 21/4/2010 Reading and following the SOP Individual user and their supervisor 21/4/2010 Record the names of those consulted when deciding on risk control measures Peter Kerr, Joe Dimauro, Jenny Phuyal, Zia Ahmad, Angela Nikolic, Robert Czolij, Craig Jackson PART B completed by: Nick Coleman Date: 22/3/2010