Factors associated with low progesterone concentrations and their
advertisement

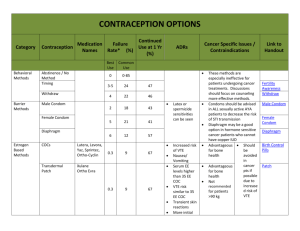
ISRAEL JOURNAL OF VETERINARY MEDICINE FACTORS ASSOCIATED WITH LOW PROGESTERONE CONCENTRATIONS AND THEIR RELATION TO LOW FERTILITY OF LACTATING DAIRY COWS Wolfenson D. Department of Animal Science, Faculty of Agriculture, the Hebrew University, Rehovot 76100, Israel Introduction In the last two decades our understanding of reproductive biology in cows has increased significantly. This was made possible due to intensive use of methods such as ultrasonography, in vitro maturation and fertilization of oocytes, gene expression technology, and other techniques. However, despite these innovations, dairy cow fertility did not improve over the years. Low fertility becomes a major limiting factor in efficiently managed dairy farms in both developed and underdeveloped countries. Several factors are associated with low fertility of the dairy cow; among them is the low concentration of progesterone in the circulation (Santos et al., 2004). Low progesterone may affect reproductive processes either before or after insemination. The situation has been aggravated in recent years because achieving a continuous rise in milk production is associated with a gradual decline in plasma progesterone concentrations, consistent with the negative relationship between milk production and progesterone concentration (Lucy et al., 2001). This relationship may be attributed to accelerated progesterone metabolism in the liver. Low concentrations of progesterone in the cycle preceding artificial insemination (AI) have been associated with the persistent follicle syndrome, which has been shown to involve higher than normal pulsatile LH secretion and early maturation of the oocyte in the preovulatory follicle. Insemination following ovulation of persistent follicles resulted in retarded embryos development (Revah and Butler, 1996). It has been shown that the conception rates of cows inseminated following ovulation of a persistent follicle were considerably lower than those in normal cows (Santos et al., 2004). Suboptimal luteal function after AI could be associated with either low secretion of suboptimal amounts of progesterone or with early luteal regression. The latter situation ultimately results in embryo death. The effect of low concentrations of progesterone post AI on fertility is a controversial issue. Progesterone is essential for the maintenance of pregnancy, however, according to Lucy (2001), the minimum concentrations of progesterone required for maintaining pregnancy are not known. Nevertheless, several studies show low progesterone concentrations to be associated with low fertility. For example, Mann and Lamming (2001) showed that low plasma progesterone concentrations and a delay in the rise of luteal phase progesterone postovulation were associated with retarded embryo growth and low secretion of interferon-t on day 16 post AI. Retarded embryos on day 16 may eventually die, or low interferon-t could lead to early luteal regression and secession of pregnancy. Indeed, low progesterone has been shown to be associated with enhanced secretion of PGF2a, which may induce luteolysis of the corpus luteum (CL) and termination of pregnancy (Santos et al, 2004). This minireview deals with three different aspects involving low progesterone concentrations. The first aspect examined the effects of low preovulatory LH surge concentration on CL growth and progesterone secretion. The second aspect examined the debatable effect of summer heat stress on progesterone secretion. The third aspect examined the consequences of the delayed effect of low progesterone concentrations in one estrous cycle on PGF2a secretion in the subsequent cycle. The data presented have been generated in our laboratory in recent years. Preovulatory LH surge and post-ovulation progesterone concentrations Preovulatory LH surge induces a chain of events in the ovulatory follicle that are essential for the formation of a normally active CL. More specifically, LH surge induces a sharp decline in the production of P-450 aromatase and a sharp increase in the production of P-450-scc and 3b-HSD in the granulosa cells, resulting in a drop of estradiol production and an increased production of progesterone in the ovulatory follicle. The possible relation between low LH surge and the formation of suboptimal CL has not been studied extensively. Low progesterone curves could result from low LH surge, which could be associated with suboptimal luteinization of the growing CL, following ovulation. Interestingly, this relationship was found in primates (ZelinskiWooten et al., 1997). Studies in cows (Ambrose et al., 1998) showed that an induced low LH surge was associated with low post-ovulation plasma progesterone concentrations. To shed further light on these issues, we have studied the effect of different preovulatory LH surge levels on progesterone curve and CL development following ovulation (Less et al., 1998). Briefly, cyclic lactating Holstein cows were synchronized. Following estrus, the presence of dominant follicles and CL was verified by ultrasonography on day 7 of the cycle. The CL regressed following PGF2? injection, and 40 h later, on day 9, the cows received a dose of hCG (5000 IU) or a dose of GnRH analogue (Receptal, Buserelin) to induce LH surge. The doses were 2 µg, 4 µg, 8 µg (the recommended commercial dose),and 16 µg, or 2 injections of 8 µg Buserelin given 90 min apart. Blood samples were taken frequently to characterize the LH surge. Cows in all the treatments ovulated. The average LH duration was 4 h and the LH surge peak ranged from 13 to 46 ng/ml. The peak value, the mean curve, and the area under the LH surge curve were lower (P<0.05) in the 2-µg Buserelin dose than in the other groups (Figure 1). In correlation with these data, the peak progesterone value of the induced cycle was lower in the 2-µg Buserelin group than in all other Buserelin-treated groups (Figure 2). The mean and the area under the curve of progesterone were lower in the Buserelin groups than in the hCG group. Notably, 2x8 µg Buserelin treatment did not induce a wider LH surge, and the progesterone curve was not different from that of 8-µg treatment. The CL diameter did not differ between the groups. Importantly, this study showed that a low LH surge induced a lower plasma progesterone profile, and that the hCG injection which maintains a longer period of biological activity, induced a higher progesterone curve. To support this in vivo finding, we (Less et al., 1998; Biger et al., 2000) examined the steroidogenic capacity of granulosa and thecal cells exposed to different LH surges at the beginning of luteinization. Luteinized granulosa cells exposed to low LH 'surge' doses on day zero of culture, produced at the end of luteinization on day 8 of culture, 2.5 times less progesterone than cells exposed to high LH 'surges'. Similarly, lueinized granulosa cells exposed to a long duration LH 'surge' on day zero of culture produced more progesterone at the end of luteinization than cells exposed to a shorter LH 'surge'. Collectively, the above studies indicate that a preovulatory LH surge of adequate size is required for the development of a fully functional CL. Effect of chronic, summer heat stress on progesterone secretion Suboptimal progesterone secretion is a possible cause of low fertility of dairy cows during summer heat stress. This issue is controversial, however, and various studies have found progesterone levels under heat stress to be higher, lower, or similar to those under cool conditions (Wolfenson et al., 2000). The divergence among these findings arises from the fact that most short-term, acute experiments did not reproduce the responses obtained in long-term, chronic, seasonal studies. Holstein cows in winter and in summer, at 60 to 80 days post-partum, yielding 49.0 ± 2.4 and 45.1 ± 2.2 kg milk/day, in winter and summer, were used in the study (Wolfenson et al., 2002). Concentrations of plasma progesterone were significantly higher in winter than in summer (Figure 3). No effects of milk yield or parity were detected. Progesterone concentrations in the early days of the cycle were similar in both seasons; however, during the mid-luteal phase they were 1.5 ng/ml higher, on average, in winter than in summer (P < 0.05). To support the in vivo findings of a higher progesterone curve in winter, we examined seasonal differences in progesterone production by evaluating granulosa cells and thecal cells that were collected from first wave dominant follicles and were luteinized in vitro during the winter or summer. At the end of 9 days of luteinization, progesterone production by luteinized granulosa cells was twice higher (P=0.10), and that of luteinized thecal cells was three times higher (P<0.05) in winter than in summer. Importantly, the study demonstrated that heat stress-induced damage to follicular function was carried over to the subsequently formed CL, a damage that was noted in the granulosa-derived, large luteal cells, but it was more pronounced in the thecal-derived, small luteal cells. It was concluded that in most studies in which cows have been chronically heatstressed during the summer, the concentrations of progesterone decreased compared with normothermic cows. In contrast, in most studies in which cows were acutely exposed to severe heat stress (for example, solar radiation or very high temperatures in environmental chambers), the progesterone concentrations either increased or unaltered. Delayed effect of low progesterone on PGF2a secretion on the subsequent cycle It is well established that the episodic secretion of PGF2a, which induces luteolysis, is controlled by oxytocin binding to its uterine endometrial receptors (Geisert et al., 1994), and that progesterone controls the timing of the development of these oxytocin receptors. Low progesterone concentrations post-insemination are thought to be associated with high uterine secretion of PGF2a, which may interfere with pregnancy recognition and result in embryo loss (Mann et al., 1999). However, low fertility in cattle has also been shown to be related to low progesterone concentrations in the cycle preceding insemination. We investigated a possible delayed effect of low progesterone in one cycle on uterine responsiveness to an oxytocin challenge, as expressed in PGF2a secretion in the subsequent cycle (Shaham et al., 2001). We used untreated cows to represent high progesterone treatment. These cows were compared with their counterparts, which exhibited low and ascending plasma progesterone concentrations that were achieved by manipulating endogenous progesterone secretion of the CL (Figure 4). Following oxytocin challenge on day 16 of the subsequent cycle, 18 days after progesterone treatments had ended, the increases in circulating PGF2a metabolite (PGFM) concentrations in the low progesterone group were markedly higher than those in the high progesterone group (Figure 5). The results indicate that low progesterone concentrations during an estrous cycle have a delayed stimulatory effect on uterine responsiveness to oxytocin during the late luteal phase of the subsequent cycle. The resulting increase in PGF2a secretion may interfere with luteal maintenance during the early stages of pregnancy. Summary Low progesterone secretion resulting from the formation of a suboptimal CL is a major cause of low fertility of dairy cows. The two major causes of low fertility, summer heat stress and negative energy balance, are associated with low progesterone secretion. Nevertheless, the positive results in one study in which exogenous progesterone was added, have not been confirmed in other studies. Thus, establishing optimal hormonal treatment to improve fertility needs further systematic experimentation. Figure legends Figure 1: Concentration of LH surges in plasma following the injection of different doses of GnRH analogue (Buserelin) 40 h after PGF2a injection. The curve of the LH surge induced by 2 mg Buserelin was lower (P<0.05). Figure 2: Concentrations of plasma progesterone following ovulation induced by hCG or different doses of GnRH analogue. The mid luteal phase level of progesterone was lower (P<0.05) in the curve induced by the lowest GnRH dose (2 mg Buserelin) than in other treatments. Figure 3: Plasma progesterone concentrations during the estrous cycle of cows in winter (•) were lower (P<0.05) than those in the summer (°). Figure 4: Plasma progesterone concentrations during (a) the treated oestrous cycle, when low and high progesterone concentration curves were induced; (b) the subsequent cycle, when uterine oxytocin challenge was induced on day 15 of the cycle. Untreated cows served as the high progesterone group (D); low progesterone concentrations in cycle 1 were achieved by three consecutive injections of PGF2a at 12-h intervals starting on day 3 of the cycle (D). Means DSEM. Figure 5: Plasma concentrations of PGFM determined from 1 h before to 3 h after oxytocin challenge (100 iu.; i.v. injection) on day 15 of the subsequent cycle, in cows exposed to high (D) or low (D) progesterone concentrations in the previous cycle. Means ±SEM. P<0.05. Link Bar 0 References 1. Ambrose J. DMF Pires, F Moreira, T Diaz, M Binelli, and WW Thatcher. Influence of deslorelin (GnRH-agonist) implant on plasma progesterone, first wave dominant follicle and pregnancy in dairy cattle. Theriogenology 50:1157-1170, 1998. 2. Biger E, D Wolfenson, R Mamluk, N Levi, Y Graber, and R Meidan. Effects of LH 'surge' patterns on the steroidogenic capacity of bovine follicular cells luteinized in vitro. 14th Int. Cong. Anim. Reprod. Stockholm, Abst. 1:27, 2000. 3. Geisert RD, EC Short, and GC Morgan. Establishment of pregnancy in domestic farm species. In: Embryonic Mortality in Domestic Species pp 23-51 Eds MT Zavy and RD Geisert. CRC Press, Boca Raton, FL, 1994. 4. Less M, D Wolfenson, A Shaham-Albalacy, Z Roth, and R Meidan. Effects of LH surge pattern on progesterone secretion during development of the corpus luteum and in vitro luteinization of granulosa and theca cells in cows. Ann. Meeting, Soc. for the Study of Fertility. Glasgow, Abst. 76, 1998. 5. Lucy MC. Reproductive loss in high-producing dairy cattle: where will it end? J. Dairy Sci. 84:12771293, 2001. 6. Mann GE, and GE Lamming. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction 121:175-180, 2001. 7. Mann GE, GE Lamming, RS Robinson, DC Wathes. The regulation of interferon-t production and uterine hormone receptors during early pregnancy. J. Reprod. Fertil. Supplement 54:317-328, 1999. 8. Revah I, and WR Butler. Prolonged dominance of follicles and reduced viability of bovine oocytes. J. Reprod. Fertil. 106:39-47 1996. 9. Santos JEP, WW Thatcher, RC Chebela, RLA Cerria, and KN Galvãoa. The effect of embryonic death rates in cattle on the efficacy of estrus synchronization programs. Anim. Reprod. Sci. 82-83:513535, 2004. 10. Shaham-Albalancy A, Y. Folman, M. Kaim, M. Rosenberg, and D. Wolfenson. Delayed effect of low progesterone on bovine uterine prostaglandin F2? secretion in the subsequent oestrous cycle. Reproduction 122:643-648, 2001. 11. Wolfenson D, Z Roth , and R Meidan. Impaired reproduction in heat-stressed cattle: basic and applied aspects. Anim. Reprod. Sci. 60-61:535-547, 2000. 12. Wolfenson D, H Sonego, A Bloch, A Shaham-Albalancy, M Kaim, Y Folman, and R Meidan. Seasonal differences in progesterone production by bovine luteinized thecal and granulosa cells. Dom. Anim. Endocrinol. 22:81-90, 2002. 13. Zelinski-Wooten MB, JS Hutchinson, I Trinchard-Lugan, DL Hess, DP Wolf, and RL Stouffer. Initiation of periovulatory events in gonadotrophin-stimulated macaques with varying doses of recombinant human chorionic gonadotrophin. Hum. Reprod. 12:1877-1885, 1997.