DENSITY AND WATER

WC-14: DENSITY AND WATER
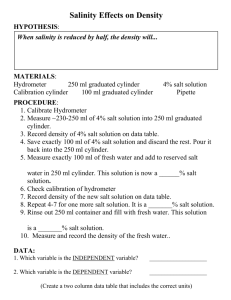
MATERIALS LISTS:
IN KIT
Jar of fresh water
Jar of salt water (add salt until it will not dissolve any more)
Red food coloring
Jar of red colored salt water
2 beakers
Plastic jug of water
Plastic ball
Lead ball the same size as the plastic ball
A few feathers
Water molecule models (container to store the molecules)
Water tray
Turkey baster
Plastic boat that will sink when full of water
TO BRING FROM HOME
*Two fresh eggs
*Several ice cubes
*Prepare these items at home. Also test both eggs to make sure that each will float in the salt solution, and each will sink in the fresh water. Use those two eggs in the classroom demonstration
Directions for making salt water:
Fill the salt water jar 3/4 full of water.
Add salt and stir until the water will not dissolve any more salt.
Stop at this point (you may see a little undissolved salt on the bottom of the jar – a little salt is OK). Put the lid on the jar.
To test for saturation, float both fresh eggs on the solution. If the eggs do not float, more salt is needed.
Add several drops of red food coloring only in one, other clear for egg.
DENSITY AND WATER WC – 14 page 1
DENSITY AND WATER
AUGUST 1999 WC – 14
OBJECTIVES:
To explain the concept of density, the relationship between ice and liquid water, fresh and salt water, and the relationship between density and substances dissolved in water
GRADE LEVELS: 3 – 6
FOCUS WORDS: Mass, weight, volume, density, molecule, brine, salinity, buoy, and float
BACKGROUND INFORMATION:
The density of material is the ratio of its mass and volume. We can calculate density using the formula: Density= Mass/Volume
Since mass is usually expressed in grams and volume in cm.
3 , density is expressed in gm/cm.
3
We can compare densities by relating the unknown density of a material with the known density of fresh water. The ratio of densities is called its specific gravity. The specific gravity of water is
1. If a material is more dense than water its specific gravity is greater than 1. Conversely, if the material is less dense than water its ratio is between one and zero
Example:
The weight of lead is 710 pounds per cubic foot.
The weight of fresh water is 62.5 pounds per cubic foot.
The specific gravity of lead is 710/62.5 which is 11.37.
The specific gravity of most fats is about 0.45.
We can see from this that if a material has a specific gravity of less than 1, it will float in fresh water; if more than 1 it will sink.
ACTIVITIES:
TIME NEEDED: 20–30 minutes.
SAY:
ACTION:
ACTION:
We are going to explore something called density.
Ask one student to put both hands on the desk with his/her palms up.
Place one of the balls (plastic) in one of his hands and the other ball (lead) in the other hand.
SAY: These two balls are the same size and color. They look alike.
ASK: Are they alike? Lets ask the person that is holding them.
RESPONSE: They look alike, but they are not alike; one is much heavier than the other.
SAY: The balls look alike, they are the same size, but the heavier ball is other one. denser than the
NOTE: It is not necessary to use up class time by passing the balls around for older children, but might be good for younger children.
DENSITY AND WATER WC – 14 page 2
ASK: What does it mean for something to be more dense and others to be less dense?
EXPLAIN: The lead ball is denser than the plastic ball because it has more mass packed into the same size package than the plastic ball has.
SAY: Next time you enter an elevator, look for a sign that says how many people are safe to ride in that elevator at one time.
ASK: Why do we limit the number of people in that elevator?
RESPONSE: The more people there are in the elevator, the heavier it is. If the load of people is too much, the elevator cables could break.
EXPLAIN If the density of people in the elevator is too much, the weight can be too much for the cables. The specific container of an elevator can only contain so much mass, made up of human bodies. Too much mass would put too much stress on the elevator cable.
ASK: How is the elevator like these too balls?
RESPONSE: The lead ball is heavier than the plastic ball because there is more mass within the volume of the lead ball than there is in the plastic ball.
ASK: What is mass?
RESPONSE: Molecules!
EXPLAIN: The mass of molecules packed into a substance determines its density, and thus the weight of a piece of a certain size. Lead packs molecules with a high mass tightly together and that ball is heavy, while plastic molecules, in comparison, have less mass, less density, and the weight of that ball is less than the lead ball.
ASK: What is heavier, a pound of lead or a pound of feathers? Is this the right way to ask that question?
SAY: They both weigh the same; one pound. But, what about their relative mass and the volume each mass would occupy?
ASK: How big is a pound of lead?
RESPONSE: This fishing sinker weighs one pound. It is just a little over one inch in diameter
(round).
ASK: How big is a pound of feathers?
RESPONSE: Feathers come in different densities so it depends upon what bird the feathers came from, but you could stuff a regular bed pillow with a pound of most feathers.
SAY: From this we can see the differences between weight, mass and density.
ACTION: Arrange the water molecules so they represent frozen water. The molecules should form a hexagonal pattern.
SAY: Remember that in liquid water the molecules are sliding past each other but when they freeze they move farther apart and then lock in place in a crystalline pattern like the model we have here.
DENSITY AND WATER WC – 14 page 3
ASK: What do you think would happen if I tried to fill a cup with these little molecules?
ACTION: Separate the molecule models and place them in a cup or dish. Few will fit, but that's OK.
ASK: What would happen if I tried to fit the model of frozen water in the cup or dish?
DENSITY AND WATER WC – 14 page 4
ACTION: Rearrange the molecules into the ice arrangement, and try to place it in the dish
RESPONSE: It doesn't fit.
SAY: When water freezes, it expands. That is, it becomes less dense. When it becomes less dense it gets bigger than it was when it was liquid, but its mass remains the same even though the volume it occupies has increased. It can only displace its own weight in water; thus it floats.
ASK: What will happen if I drop an ice cube in the water; will it float or sink?
ACTION: Float an ice cube in water.
SAY: As we expected, it floats. From this we can see that ice is less dense than the water that it came from. If ice was more dense than water it would sink to the bottom.
SAY: Salt is denser than water. If we put salt in water it will sink to the bottom. It will also dissolve.
ASK: If we dissolve salt—the more dense substance—into water—the less dense substance—will the salty solution become more dense, less dense, or will it not change?
RESPONSE: It will become more dense.
SAY: I have two fresh eggs here. I also have one jar of fresh water, and one of salt water.
If I put one egg in the fresh water and one in the salt water, what difference would you expect? State your hypothesis, scientific guess.
RESPONSE: The fresh egg in the fresh water should sink, it is more dense than the water. The fresh egg in salt water should float, it is less dense than the salt water.
ACTION: Place the first egg in the fresh water. Give the class a little time to think about this.
Then place the other egg in the salt water.
SAY: Now we have completed this experiment. What do we know about the fresh water, the salt water, and the eggs?
NOTE: It is not necessary to remove the eggs from the jars if other classes will follow this one.
SAY: Have you ever gone swimming in the ocean? It is easier to float in the ocean and harder to swim underwater. This is because ocean water (salt water) is more dense than fresh water. The ocean water buoys (floats) us up more.
SAY: The density of the human body is very nearly the same as that of ocean water but slightly less.
ASK: How do SCUBA divers manage to stay under water?
RESPONSE: They wear lead weights.
EXPLAIN: The density of those lead weights added to the density of the diver's body balances the total density with the density of the ocean water. Without those weights, the diver's density would be slightly less than that of the water, and so he would float on the surface.
DENSITY AND WATER WC – 14 page 5
SAY: The Great Salt Lake in Utah has so much salt in it that the water is so dense that a person can float in it with his head well out of the water, even with no arm or leg swimming motion.
SAY: I want to show you what happens when the salt water meets the fresh water. This experiment will give you an idea of what happens when the fresh water of a river runs into the salt water of the ocean of the Bay. It will also show you what happens when the tide pushes salt water up a fresh water river.
ACTION: Pour about 2 inches of fresh water into a jar or beaker.
Then using the turkey baster, suck up some red salt water from the red salt water jar
.
SAY : We colored the salt water red so that you could see the layers. I am going to slowly add this red salt water to the clear fresh water. What do you think will happen to the red salt water? Will it mix with the fresh water, float on top or stay at the bottom?
First, think about what we talked about. Which is denser, salt or fresh water?
ACTION : Slowly squeeze the red salt water from the baster with the tip at the bottom of the beaker or jar. Hold with finger to get it there without colored water leaking. Can you think of a place around here where salt water and fresh water mix?
EXPLAIN: This layering is very important to fish and other creatures that live in San Francisco
Bay. Certain fish can survive in the lower saltier waters but not in the upper less salty layers. Other creatures must stay in the less salty layer.
EXPLAIN: The different densities of water in the ocean form underwater currents or streams of moving water that affect our weather.
OPTIONAL: (do if there is time)
ASK: Why does a boat float in water?
ACTION: Float the boat in the dish of water.
SAY: The material of the boat is usually more dense than the water it is floating on, but still it floats. The boat is shaped in such a way that it can displace its weight of water before it takes water in. If the boat leaks, it would get heavier as the water flows in. It would displace more water to balance its new total weight. If the leak is not plugged, more water would flow in. The weight and mass of the boat would continue to increase. It would displace more water until the water comes to the deck of the boat. Now the water can run in without interference, and if the boat, full of water, is more dense than the water it now displaces it will sink. However, if it is a plastic boat, full of water, it will still float because the plastic is less dense than the water.
ACTION: With the turkey baster, slowly drop water into the boat.
ACTION: Have the children watch the boat float deeper and deeper in the water until it finally sinks.
DENSITY AND WATER WC – 14 page 6
p
Bo at f loat ing o n w at er
Th e bo at w eighs 5 00 ki lo grams
Th is is th e w at er th e boat d isp laces.
Th is amount o f w at er weigh s exactl y th e same as th e b oat
DENSITY AND WATER WC – 14 page 7
The water ex pand ed this much
This is the test tube full to the
top with liquid water
This is the s ame t est tu be with t he same wate r in it
except n ow the water is
frozen t o ice.
DENSITY AND WATER WC – 14 page 8