frequently asked questions about commercial support
advertisement

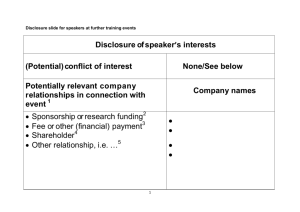
FREQUENTLY ASKED QUESTIONS ABOUT COMMERCIAL SUPPORT FOR CONTINUING EDUCATION ACTIVITIES DISCLOSURE Is it necessary to have a Disclosure Form on file for every planner or faculty member who participates in a CE activity, even if the program is not receiving any commercial support or the speaker is not a physician? Yes, a disclosure statement is required from every planner, activity director and speaker. This includes pharmacists, physicians, other professionals and para-professionals and is required regardless of the source of funding for the event or the topic(s). The intent of this policy is not to prevent anyone with a potential conflict of interest from planning an activity or making a presentation. It is intended that any potential conflict of interest be identified openly and resolved in advance of the activity, so that the listeners may form their own judgments about the activity or presentation with full disclosure of the facts. Must I have a planner or faculty member fill out a new Disclosure Form each time he/she plans an activity or presents at a CE activity? Generally yes, planners or faculty must complete new disclosure forms each time they speak or are involved in the planning of a new activity. The only exception is for faculty/planners involved in a non-clinical activity (i.e. legal issues). For faculty/planners involved in a nonclinical program, the same disclosure form may be used for up to one year (subject to approval from the Office of CPE). You must ensure that a signed copy of the disclosure form is included in the CE file for each speaker and planner for each CE activity and document that disclosure was made to participants at each CE activity. Does disclosure apply only to relationships with pharmaceutical companies or does it also apply to relationships with other companies? If there is any possibility that a relevant financial relationship exists with a company or organization that could bias the planner or speaker in any way, it is essential that this relationship be disclosed (e.g. a partner in a law firm making a presentation regarding a legal topic should disclose his relationship with the law firm). What is a commercial interest? A commercial interest is any entity producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients. Are speakers allowed to discuss off label or investigational uses of a medication in a CE presentation? Discussion of off-label uses are certainly allowed in CE activities. However, providers are no longer required to have a mechanism in place to ensure that off-label or investigational uses are disclosed as such. The ACCME adopted content validation statements in 2002 that are expectations of providers with regard to any recommendations for clinical care. Specifically, Revised 12/10 S:OCPE\joint policy and procedure manual\FAQ Commercial Support 1 “all the recommendations involving clinical medicine in a CME activity [are] based on evidence that is accepted within the profession of medicine as adequate justification for their indications and contraindications in the care of patients. All scientific research referred to, reported or used in CME in support or justification of a patient care recommendation [conforms] to the generally accepted standards of experimental design, data collection and analysis.” You are responsible for communicating these expectations to your speakers/faculty. What constitutes a “relevant financial interest”? In most cases, the individual should consider what others might perceive to be a potential conflict of interest, such as ownership of stock, research grants, serving on a speaker’s bureau, or receiving consulting fees. What are my options for ways to disclose financial interests to the audience? Disclosure may be made in the handouts, as a power point slide at the beginning of an activity/presentation or verbally from the podium. There must be documentation in the CE file that disclosure was made prior to the faculty member’s presentation. Disclosure should include: a) name of company and nature of the relationship if relationships are present, or b) state that no relationships are present if that is the case. With respect to the documentation of verbal disclosure at CE activities: 1. a representative of the provider who was in attendance at the time of the verbal disclosure must attest, in writing: a) that verbal disclosure did occur; and b) itemize the content of the disclosed information (Standards for Commercial Support); or that there was nothing to disclose (Standards for Commercial Support). 2. The documentation that verifies that adequate verbal disclosure did occur must be completed within one month of the activity. I have requested that a planner/speaker return a Disclosure Form several times but have not been able to get one. What should I do? An individual who refuses to disclose relevant financial relationships will be disqualified from being a planning committee member, a teacher, or an author of CE, and cannot have control of, or responsibility for the Education, management, presentation or evaluation of the CE activity. (Standards for Commercial Support) EXHIBITORS AT CE ACTIVITIES Must I have a Commercial Support Agreement Form signed by an exhibitor at my CE activity? No. As exhibits are an arrangement for which the company receives something tangible in return for their exhibit fee, it is not the same as an unrestricted educational grant. Revised 12/10 S:OCPE\joint policy and procedure manual\FAQ Commercial Support 2 What limitations are there on exhibits at a CE activity? Exhibits must be located in a different room from where the educational program is being conducted. No promotional materials from a commercial supporter may be distributed in the meeting room, including pencils, pads of paper, or company newsletters. There must be a clear separation of marketing activities from the educational program. May exhibitors and/or pharmaceutical reps attend a CE activity? Yes, they may attend but may not engage in sales activities while in the room where the activity takes place. It is up to you if you wish to charge the individual the standard registration fee to attend the event. UNRESTRICTED EDUCATIONAL GRANTS If a pharmaceutical company is giving you support for an activity, who makes the decision about the amount of the honorarium? Decisions about the amount of honorarium to be paid are the responsibility of the planning committee/activity director. You should determine what constitutes a “reasonable” honorarium for invited speakers and negotiate directly with the speaker. If the speaker does not wish to accept the amount offered, an alternative speaker should be identified. It is NOT appropriate for the pharmaceutical company providing an educational grant to determine the size of the honorarium. May I use educational grants to support the cost of meals or other social events associated with my CE activity? The AMA Guidelines on gifts to physicians state that social events should be conducive to discussion and exchange of ideas. The cost of these social events should be “modest” and the number of hours devoted to the educational activity should substantially outweigh the number of hours of the social event. Social events, such as dinners in conjunction with a CE activity, should be paid for by those planning the program and not directly by a pharmaceutical representative. Are funds received from organizations that are not pharmaceutical companies considered to be commercial support? A commercial supporter is defined as any entity providing funds or resources in support of CE activities with the exception of governmental entities. Therefore, funds received from law firms, hospitals (excluding your own dept’s funds), the Robert Wood Johnson Foundation, or the American Cancer Society are considered to be commercial support. Funds received from the Ohio Department of Health or the Akron Metropolitan Housing Authority are not considered to be commercial support. Revised 12/10 S:OCPE\joint policy and procedure manual\FAQ Commercial Support 3 What is the best way to acknowledge a company who provided an educational grant? Accrediting agencies require that participants be informed of who provided educational grants in support of the activity. Acknowledgment may state the company’s name, mission and areas of clinical involvement. The company’s logo and slogans may also be included if they are not product promotional in nature. Acknowledgment may be made on the front of the handouts, in a slide at the beginning of an activity, from the podium, on an easel by the entrance door(s) to the meeting room, or in the conference brochure. The method of acknowledging commercial support must be documented in the CE file. Note that those providing commercial support are NOT to be called “sponsors” as the sponsor of a program refers specifically to the accredited CE provider. Can a commercial supporter give physicians invitations to your CE activity? Yes, but only the accredited provider may authorize a commercial supporter to disseminate information about a CE activity to the medical community. The content of the promotional material is the responsibility of the provider. Is it acceptable for a joint sponsor of a CE activity to receive the commercial support funds in place of the accredited provider? Yes, the accredited provider may delegate this responsibility to the joint sponsor as long as the Letter of Agreement is between the accredited provider and the commercial supporter and as long as the accredited provider maintains documentation of the funds in the CE file for the activity (signed agreement form, copies of checks received, etc). Since the new guidelines regarding commercial support went into effect for the pharmaceutical industry, I have been asked to return agreement forms designed by the pharmaceutical companies. What should I do? Accrediting agencies Standards For Commercial Support require that seven elements be included in these agreements: which CE activity is being supported, who are the funds going to and which person is responsible for the CE activity, which company is providing the support, how is the support to be used (i.e. unrestricted educational grant), what are the conditions and requirements that will be followed (i.e. limitations on company’s involvement with the program), signatures of company rep/ the person responsible for the activity, and the person in the Continuing Professional Education Office responsible for oversight of the activity. As long as this information is included in the company’s letter of agreement, it may supersede the provider’s agreement form. However, be advised that the letter of agreement is to be between the pharmaceutical company and the accredited sponsor of the CE credit (NOT between the company and the joint or co-sponsor). Revised 12/10 S:OCPE\joint policy and procedure manual\FAQ Commercial Support 4 ENSURING THAT CE CONTENT IS FAIR AND BALANCED Is there anything wrong with selecting a CE speaker from a “speaker’s list” provided by a pharmaceutical company? There is no reason to request suggestions or topics from commercial interests since it is unacceptable to act upon their suggestions. Accrediting bodies state that providers cannot receive any advice or guidance, either nuanced or direct, on the content of educational activities or on who should deliver educational content. How can you assure that a CE activity is free of commercial bias? You and your planning committee should maintain complete control over all aspects of the educational planning and implementation process, including selection of topics and speakers, and control of funding. Must generic names always be used in discussions of products and treatments? Generally, yes. In order to decrease the chances of promotional intent, generic names are preferred. In practice, it is sometimes necessary to use generic AND product names because generic names may not be recognized by participants. However, presentations must be balanced, with discussion of both benefits and risks of the treatment, as well as alternative products and therapies. May a commercial supporter pay a speaker directly? No, direct payment of an honorarium or speaker’s expenses establishes a relationship between the speaker and the company which creates the potential for bias in a presentation. In addition, direct payment of expenses is in violation of the pharmaceutical industry’s new guidelines. MISCELLANEOUS Is it okay to give a list of participants for a CE activity to the commercial supporters or exhibitors for my activity? There is no accrediting agency policy prohibiting distribution of participant lists. However, does your own organization have policies on privacy or sharing physician’s information? Distribution of names and addresses for future marketing campaigns by pharmaceutical companies may be in violation of your organization’s internal policies and/or ethical guidelines. Revised 12/10 S:OCPE\joint policy and procedure manual\FAQ Commercial Support 5