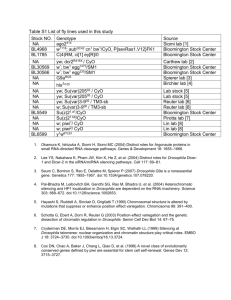
homologous genet
advertisement

The origin and evolution of a new chimeric gene in Drosophila melanogaster Ren Juan, Biological Science, Grade 2003 Directed by Zhang Huaiyu (Professor, Sichuan Agricultural University) Wang Wen (Professor, Kunming Institute of Zoology the Chinese Academy of Science) Abstract: A new chimeric gene GENE18 in Drosophila melanogaster originated about 2.5 mya ago after the divergence of the D.melanogaster species. P-element transposition which induced gene disruption was used here to create loss function lines. Through crossing with different fruit fly lines for five generations, the disrupted lines with the genotype ‘yw/Y; +/+; P[gene-]/ P[gene-]’ and ‘yw/yw; +/+; P[gene-]/P[gene-]’were selected by the phenotype with white eyes, slender body, long bristles and straight wings. And further more, the DNA amplification products showed that the disrupted lines had lost fragments longer than 2kb in the 5’region of this new gene. Some observation indicated that the individual fitness in the homologous disrupted lines was poorer than one in the heterozygous lines and the non-disrupted lines. Key words: chimeric gene, gene duplication, exon shuffling, homologous line, EP 1 Introduction Evolution is an old topic since Darwin’s “the origin of species” and evolutionists used variant tools to verify their hypotheses about evolution. As the whole genomes of various organisms are sequenced scientist is gradually focusing on the evolutionary history of new genes in genomes using molecular evolution tools [1] ~ [2]. Modular organisms are the pioneers which are put into the river of evolution, such as yeast, worm and fruit fly, etc. With the clear genetic background and the convenient cultivation fruit fly becomes the best modular organism to investigate the origin of new genes. The study of ancient genes highlights the antiquity and general importance of some mechanisms of gene origination, and recent observations on young genes at early stages have unveiled unexpected molecular and evolutionary processes [3]. Having avoided the missing data newly evolved genes are the keys to unlock the 1 evolutionary mystery. Evolutionists expect to explore laws of evolution with molecular evolution tools. New genes contribute a lot to the high diversity of species. It is fantastic to study the origin of new genes that originated recently as well as to disclose the mystery of their evolutionary processes. New genes provide more information than old ones which lost most of the data during long-term evolution. Recently, a new chimeric gene GENE18 was screened in the genome of D.melanogaster using cDNA alignments. It is a non-coding gene in the third chromosome of D.melanogaster created by partial duplication and exon shuffling [4]. The evolutionary process has been clarified using evolution computation method (Zhan Zubing, etc. not published ). However, as it is referred above, it is not sufficient for understanding the process during evolution. And it was argued that partial duplication in conjunction with gene fusion and shuffling events can lead to an immediate acquisition of a novel function conferring a great selective advantage [5]. The aim of this investigation was to explore some novel function of this non-coding gene GENE18 which might enrich the understanding of the evolutionary process of this novel gene. The origin and evolution of GENE18 As it was mentioned, there were several mechanisms to create new genes, especially gene duplication and exon shuffling [6] . Using cDNA of D.melanogaster to align with the whole genomes of D.melanogaster, D.simulans, D.sechellia, D.yakuba and D.erecta, GENE18 was found only in D.melanogatster [7]. And the structure of this gene was clarified using BLAST and DNA sequencing. It was created by exon shuffling of the two partial duplications of GENE17 and GENE40 respectively which were in chromosome III as well as this new gene after the divergence of D.melanogaster 2.5 mya ago(Figure1the evolution tree of D.melanogaster species). There are two adjacent genes to this chimeric gene. There was one deletion in the first exon and thirty-one deletions in the third exon which came from GENE17. And also there were four deletions in the third exon that came from GENE40. Figure 2 was the formation and structure of GENE18. For the reason that there were several pre-terminated stop codons and frame shift mutation in the ORF contrasted to original two duplication fragments of the parental genes, we presumed that it was a non-coding gene which was not the same as it was annotated in the Database of NCBI and Flybase. It might be an RNA gene or a pseudo gene. But it was 2 presumed it to be an RNA gene with novel functions for three reasons. First it expressed in embryo. Second there are two isforms in male D.melanogaster while only one in female. Third it is relatively conserved to its parental genes [8] and there are only several changes in the nucleotides and deletions in sequences. Figure 1 GENE18 evolution tree Figure 2 The origin and structure of GENE18 3 Function research Recently it has been observed that RNA genes play an important role in the history of evolution [9] ~ [10]. And it is crucial to study the evolution of RNA genes in order to understand the divergence of biological diversity. For instance, an RNA gene sphinx had been detected to have something to do with the courtship of the male fruit fly [10] .Once it was knocked out the male would propose male instead of female like a gay. The new gene GENE18 has two splicing isforms in male fly but only one in female. So why did it evolve another isform? Did it have another function in males? If so, what’s the new function? What’s more, did it have some relation with its evolution process? So it needed to do the job of excision of this new gene and tried to observe the novel function to validate the guesses. Generally, gene knock-out, gene knock-down and over-expression are classic strategies to study the function of target gene. And gene knock-out and knock-down are the direct way to finish this job [9]. There are several ways to achieve gene knock-out, such as transposon-induced deficiencies and homologous recombination [11], etc; examples are P-element excision and gene targeting. For P-element excision, it is based on the fact that in most cases P-elements excise imprecisely when transposing via the P transposase [12]. Most of these imprecise excision events delete sequences from the P elements behind. However, in a reasonable proportion of excisions (~10%) flanking genomic DNA is removed by the P elements, and produces small deletions around the original P element insertion point [13] ~ [14]. The use of this approach allows to create local mutations in genes neighboring the original P insertion, e.g. in lines of the P[gene] insertions that do not have a phenotype by itself. While for targeting like ends-out and ends-in, it is complex and low efficiency to create destructed genes. For the help of Berkeley Drosophila Genome Project, there are numerous P-elements insertion lines in stock centers . We had got the insertion line EP3 of this new gene in Szeged Drosophila Stock Centre in Hungary, so it was convenient to excision the target new gene GENE18 in Chromosome 3 just using classic genetics method. Before the gene was excised what it needed to balance the three chromosomes(1,2,3) to preclude the recombination of each chromosome in case that exchange of chromosomes may affect the phenotype of the knock-out line. The balance line “yw/yw; sp/cyO; MKRS/TM6B” was usually used during gene deletion. Then “yw/yw; sp/cyO; △2-3, Sb /TM6B” line was used for the transposition of EP. Once it had 4 got the excision of GENE18 we must change the chromosome back to wild type. So the phenotype of gene excision line wouldn't be affected by the balancer. Using classic genetics and modern molecular tool we designed a scheme to gain a homologous line with disrupted gene to clarify the novel function of GENE18. We tried to find out whether it was an RNA gene with special function such as sphinx. So we used P-element excision to knock out this new gene to find out the truth. 2 Materials and methods 2.1 Disruption of gene 2.1.1 Materials Three lines of D.melanigaster: EP3 (yw/yw; +/+; P[gene]/P[gene]), yw/Y; sp/cyO; MKRS/TM6B, yw/yw; sp/cyO; △2-3, Sb/TM6B. 2.1.2 Methods 1st generation (balance) MM (many males) and mm (many females) yw/Y; +/+; P[gene]/P[gene] × yw/yw; sp/cyO; MKRS/TM6B ↓ yw/yw; +/cyO; P[gene]/TM6B yw/Y ; +/cyO; P[gene]/TM6B yw/yw; +/cyO;p[gene]/MKRS yw/Y ; +/cyO; p[gene]/MKRS yw/yw; +/sp; p[gene]/TM6B yw/Y ; +/sp; p[gene]/TM6B yw/yw; +/sp; p[gene]/MKRS yw/Y ; +/sp; p[gene]/MKRS ‘yw/Y; +/cyO; P[gene]/TM6B’ lines were chosen for the next generation. 2nd generation balancing (here occurs the excision) MM and mm yw/Y; +/cyO; P[gene]/TM6B × yw/yw; sp/cyO; △2-3,Sb/TM6B ↓ yw/Y; +/sp; P[gene-]/△2-3,Sb yw/yw; +/sp; P[gene-]/△2-3,Sb yw/Y; +/sp; P[gene-]/TM6B yw/yw; +/sp; P[gene-]/TM6B yw/Y; +/cyO; P[gene-]/△2-3,Sb yw/yw; +/cyO; P[gene-]/△2-3,Sb 5 yw/Y; +/cyO; P[gene-]/TM6B yw/yw; +/cyO; P[gene-]/TM6B yw/Y; sp/cyO; P[gene-]/△2-3,Sb yw/yw; sp/cyO; P[gene-]/△2-3,Sb yw/Y; sp/cyO; P[gene-]/TM6B yw/yw; sp/cyO; P[gene-]/TM6B ‘yw/Y; +/cyO; P[gene-]/△2-3, Sb and yw/yw; +/cyO; P[gene-]/△2-3,Sb’ lines were chosen for the next generation. 3rd generation M (single males, mosaic eye color) and mm (TM6B virgin female) yw/Y; +/cyO; P[gene-]/△2-3,Sb × yw/yw; sp/cyO; MKRS/TM6B ↓ yw/Y; +/cyO; P[gene-]/TM6B yw/yw; +/cyO; P[gene-]/TM6B yw/Y; +/sp; P[gene-]/TM6B yw/yw; +/sp; P[gene-]/TM6B yw/Y; +/cyO; P[gene-]/MKRS yw/yw; +/cyO; P[gene-]/MKRS yw/Y; +/sp; P[gene-]/MKRS yw/yw; +/sp; P[gene-]/MKRS yw/Y; +/cyO; △2-3,Sb /MKRS yw/yw; +/cyO; △2-3,Sb /MKRS yw/Y; +/sp; △2-3,Sb /MKRS yw/yw; +/sp; △2-3,Sb /MKRS yw/yw; +/cyO; P[gene-]/△2-3,Sb × yw/Y; sp/cyO; MKRS/TM6B ↓ yw/Y; +/cyO; P[gene-]/TM6B yw/yw; +/cyO; P[gene-]/TM6B yw/Y; +/sp; P[gene-]/TM6B yw/yw; +/sp; P[gene-]/TM6B yw/Y; +/cyO; P[gene-]/△2-3,Sb yw/yw; +/cyO; P[gene-]/△2-3,Sb yw/Y; +/sp; P[gene-]/△2-3,Sb yw/yw; +/sp; P[gene-]/△2-3,Sb yw/Y; +/cyO; P[gene-]/MKRS yw/yw; +/cyO; P[gene-]/MKRS yw/Y; +/sp; P[gene-]/MKRS yw/yw; +/sp; P[gene-]/MKRS yw/Y; +/cyO; P[gene-]/MKRS yw/yw; +/cyO; P[gene-]/MKRS yw/Y; +/sp; P[gene-]/MKRS yw/yw; +/sp; P[gene-]/MKRS ‘yw/Y; +/cyO; P[gene-]/TM6B, yw/Y; +/sp; P[gene-]/TM6B ’ lines were chosen for the next generation. 6 4th generation (backcross with TM6B) M(single male) and mm(several virgin female of TM6B) yw/Y; +/cyO; P[gene-]/TM6B × yw/yw; sp/cyO; MKRS/TM6B ↓ yw/Y; +/sp; P[gene-]/TM6B yw/yw; +/sp; P[gene-]/TM6B yw/Y; +/sp; P[gene-]/ MKRS yw/yw; +/sp; P[gene-]/ MKRS yw/Y; +/sp; TM6B / MKRS yw/yw; +/sp; TM6B / MKRS yw/Y; +/cyO; P[gene-]/TM6B yw/yw; +/cyO; P[gene-]/TM6B yw/Y; +/cyO; P[gene-]/ MKRS yw/yw; +/cyO; P[gene-]/ MKRS yw/Y; +/cyO; TM6B / MKRS yw/yw; +/cyO; TM6B / MKRS yw/Y; sp/cyO; P[gene-]/TM6B yw/yw; sp/cyO; P[gene-]/TM6B yw/Y; sp/cyO; P[gene-]/ MKRS yw/yw; sp/cyO; P[gene-]/MKRS yw/Y; sp/cyO; TM6B / MKRS yw/yw; sp/cyO; TM6B / MKRS yw/Y; +/sp; P[gene-]/TM6B × yw/yw; sp/cyO; MKRS/TM6B ↓ yw/Y; +/sp; P[gene-]/TM6B yw/yw; +/sp; P[gene-]/TM6B yw/Y; +/sp; P[gene-]/ MKRS yw/yw; +/sp; P[gene-]/ MKRS yw/Y; +/sp; TM6B / MKRS yw/yw; +/sp; TM6B / MKRS yw/Y; +/cyO; P[gene-]/TM6B yw/yw; +/cyO; P[gene-]/TM6B yw/Y; +/cyO; P[gene-]/ MKRS yw/yw; +/cyO; P[gene-]/ MKRS yw/Y; +/cyO; TM6B / MKRS yw/yw; +/cyO; TM6B / MKRS yw/Y; sp/cyO; P[gene-]/TM6B yw/yw; sp/cyO; P[gene-]/TM6B yw/Y; sp/cyO; P[gene-]/ MKRS yw/yw; sp/cyO; P[gene-]/MKRS yw/Y; sp/cyO; TM6B / MKRS yw/yw; sp/cyO; TM6B / MKRS Then ‘yw/Y; +/cyO; P [gene-]/TM6B, yw/yw; +/cyO; P[gene-]/TM6B’ lines were chosen for the next generation. 7 5th generation (brother-sister crossing) M (sing male) and m (single female) yw/Y; +/cyO; P[gene-]/TM6B × yw/yw; +/cyO; P [gene-]/TM6B ↓ yw/Y; +/+; P[gene-]/ P[gene-] yw/yw; +/+; P[gene-]/P[gene-] yw/Y; +/ cyO; P[gene-]/ P[gene-] yw/yw; +/ cyO; P[gene-]/P[gene-] yw/Y; +/+; P[gene-]/ TM6B yw/yw; +/+; P[gene-]/ TM6B yw/Y; +/ cyO; P[gene-]/ TM6B yw/yw; +/ cyO; P[gene-]/ TM6B ‘yw/Y; +/+; P[gene-]/ P[gene-] and yw/yw; +/+; P[gene-]/P[gene-]’ were the disrupted lines needed to be the next research. 2.2 Detection of the deletion lines by DNA amplification and sequencing 2.2.1 Design of primers The targeted primers, two forward primers and reverse primers obtained by the primer designer Oligo6.69. The followings were the primers we designed. And the positions of designed primers and primers mel-R1, mel-R2 and seq2R used before (Zhan Zubing, not published yet) were shown in figure3 as well as the positions of the two adjacent genes (gene1 and gene2) and the regions AD1, AD2 among these three genes. GENE18 F1 TAATTTAATGCAATCCGGTAC GENE18 F2 ACATTTTACAGCCGACGTTAC GENE18 R1 ATCACCATATTGGCGAGTTAC GENE18 R2 TTGCAATTCCTTTCCGAAGCA Figure 3 the relative positions 8 2.2.2 Detection of the excision lines 2.2.2.1 Extraction of the genome of W1118 wild type, ‘yw/Y; +/cyO; P[gene-]/TM6B’ and ‘yw/yw; +/cyO; P[gene-]/TM6B’ excision type fruit flies during the hybridization. (1) Add cooled lysis Buffer B into sample flies (be sure to mix them together). (2) Mill the flies in tubes, and then put them on ice. (3) Put the samples in 65℃ for 40 minutes. (4) Then cool the sample to room temperature, after that add RNase into them and digest at 37℃ for 40 minutes(be sure to mix them together). (5) Cool the sample to room temperature, and then mix Prot.PPT into them for 6 minutes on ice. (6) Centrifuge at 14000 rpm for 5 minutes. (7) Take the limpid liquid into new tubes, and then Centrifuge at 14000 rpm for 5 minutes. (8) Add Isopropanol into new tubes. (9) Take the limpid liquid into Isopropanol on ice, and then vortex them tenderly. (10) Centrifuge at 3000 rpm for 5 minutes. (11) Discard the limpid liquid and add 700ul 70% ETOH to wash off the Isopropanol then Centrifuge it at 3000 rpm for 3 minutes. (12) Discard the limpid liquid and dry the DNA in air for 20 minutes. (13) Add 30ul DNA Hydration Soln at room temperature for 1hr to dissolve DNA. 2.2.2.2 Perform PCR amplification to detect the deletion line with primer pairs including GENE18 F2/GENE18 R2, GENE18 F2/melR1 and GENE18 F2/melR2 respectively in W1118 and excision lines. Preparation of the PCR mix Forward primer 0.5ul Reverse primer 0.5ul dNTP 2ul 10×Buffer 2.5ul Template DNA 2ul rTaq 0.15ul H2O 17.35ul 9 Thermal cycling 94℃ 5minutes 94℃ 30seconds 56℃ 30seconds 72℃ 2minutes 72℃ 7minutes 10℃ forever 36cycles 2.2.2.3 Gel electrophoresis of the PCR products and Sequencing of the PCR products 3 Results and Discussion 3.1 Disruption of GENE18 1st generation After the first generation the gene type ‘yw/Y; +/cyO; P[gene]/TM6B’ with red eye, curl wings and long bristle markers was the right lines for the next generation. The male P-element insertion line was used to cross with the balanced line. So there wouldn't be any recombination between male and the balanced line. In this line every chromosome was balanced by balancers except the X chromosome. But there was no exchange in the next generation, for the reason that there was no recombination in male. 2nd generation In the 2nd generation the flies with curls wings, mosaic eyes and short bristles were what we needed (Figure 3 the mosaic eyes). “yw/yw; sp/cyO; △2-3, Sb/TM6B” line can provide transposase, so during the embryo there will be P-element transposition. The somatic cells and germ line cells might have excision in GENE18. For the reason that it couldn't be sure which fruit fly had the excision so mosaic eye color fruit fly with gene marker cyO and sb had been chosen to do the next generation crossing to obtain the excision lines. 10 Mosaic eyes Figure 4 the mosaic eyes 3rd generation The gene type ‘yw/Y; +/cyO; P [gene-]/ TM6B, yw/Y; +/sp; P [gene-]/TM6B’ was obtained for the next generation. In this generation the markers MKRS (short bristle) and △2-3, Sb (short bristle) were eliminated to avoid the mistakes of selection for the next generation. 4th generation Both of the gene type ‘yw/Y; +/cyO; P [gene-]/TM6B, yw/yw; +/cyO; P [gene-]/TM6B’ were right for the next generation. During this generation all the chromosomes came from flies with one parental genotype to exclude the interference of the genetic background. 5th generation Homologous gene types ‘yw/Y; +/+; P [gene-]/ P [gene-]’ and ‘yw/yw; +/+; P [gene-]/P [gene-]’ were the final target lines. These flies had long bristle, slender body and white eyes. In this generation every chromosome was homologous. They would be kept for various experiments. 3.2 Detection of the disruption lines Selection of the primers There were different combinations of the forward and reverse primers. The primers designed before (Zhan Zubing, not published yet) were also tested with the other four designed primers. 11 There were several types of matches. However, only GENE18 F2/melR2, GENE18F1/mel-R1, GENE18/seq2R, F1/mel-R2 and GENE18 F2/melR2 worked in genome of W1118. So only these five primer pairs could be used to detect excision lines. Two pairs of primers up stream and down stream of GENE18 in two adjacent regions (AD1 and AD2) were designed respectively. In that case the P-element might excise the adjacent genes (gene1 and gene2), it was necessary to exclude the additional excision of these two genes. The two pairs of primers were about 500bp away from GENE18 in the two adjacent regions. Once it could not be amplified in the fragment between these two 500bp adjacent base pairs it could be speculated that the excision was too long to destroy the adjacent genes. PCR amplification and gel electrophoresis The tested five primer pairs were used to detect the excision lines during the 5th generation. Here was part of the gel electrophoresis result of the PCR products in figure 5 with primer pairs “F1/Seq2R” in the last four better candidates. Two strips were obtained. The longer strips were the wild type gene and the shorter were the disruption gene. The contrast of the two strips indicated that there were excisions more than 2kb in one of the homologous chromosomes. And this excision didn’t contain the adjacent genes. Figure 5 Amplification patterns obtained using primer pairs F1/Seq2R to identify disrupted lines (arrowheads). Lane M, DL2000 marker; lane 1, W1118 wild type; lane 2 and 4, disrupted lines; lane 3, non-disrupted line. As it was known that the EP excised neighboring fragments imprecisely, if it excised too short to destroy the new gene or so long to destroy the adjacent genes the excised lines would be eliminated. Only the right excised lines would be kept to do the next generation cross. The 12 design of these primers was right to the need to avoid wrong selection of flies. And the capability of being amplified with the primers in AD1 and in gene indicated that the adjacent gene 1 was not destroyed. PCR products were sent to be sequenced. 4 Conclusions Classic genetics tool was used to gain the gene disrupted line which would be the further material to study the novel function of GENE18. Although sequencing is still in the progress, this gene would not express with long fragments (about 2kb) loss in the 5’ region and it could be presumed what would happen with the information Zhan provided [15] . According to his observation of the EP3 line, the fitness of this line with homologous P-element insertion chromosomes might be poorer than the heterozygous lines with only one P-element insertion chromosome. And the new gene didn't express in the homologous insertion lines. This means that the new gene was disrupted by the EP insertion. Maybe the new gene was just in the pathway of some function mechanism [16]. However, it needs to do one more generation crossing to verify whether the result answered for Mendel's law. And for the detailed function, it still needed to be explored by further experiments like using gene chips to analyze the expression spectrum. During this experiment, the major problem was the selection of red-eye flies in the first generation and mosaic-eye ones in the second generation. The red color was a little weak in the crossed lines. So it needs more effort to differentiate the red eye and mosaic eye within white eye. Nevertheless the plan was finished and the homologous disruption lines were gained. It paves the way for the next step of analysis. Acknowledgement My thesis was finished in Key Laboratory of Cellular and Molecular Evolution of Kun Ming Institute of Zoology The Chinese Academy of Science. Thanks to Mr. Wang who gave me the guidance and opportunity. And thank very much to Zhan Zubing’s patient help. During this period I learnt skills and knowledge about the investigation of molecular evolution. At the same time, I want to own my thanks to Mrs. Yu in Yunnan University, Ding Yun, Zhao Ruoping, 13 Zhang Yue, Li Xin, Li Dan, Zhou Qi and Dong Yang of MP I. Thanks to their friendly help. At last, thanks for guidance of Mrs. Zhang huaiyu and the help of Xu Guochao in Sichuan Agricultural University. References [1]. Yu, H., Jiang et al. Origination and evolution of a human-specific transmembrane protein gene, c1orf37-dup. Human Molecular Genetics. 2006 [2]. Long Manyuan et al. Natural selection and the origin of jingwei, a chimeric processed functional gene in Drosophila. Science.1993 [3]. Long Manyuan et al. The origin of new genes: glimpses from the young and old. Nat Rev Genet. 2003 [4]. Wang, Wen et al. Duplication-degeneration as a mechanism of gene fission and the origin of new genes in Drosophila species. Nature Genet. 2004 [5]. Patthy, L. Protein evolution. Blackwell Science Ltd., Oxford. 1999 [6]. Arguello, J.R et al. The Recent Origination of an X-linked Testes-specific Chimeric Gene by Illegitimate Recombination in Drosophila.PLoS Genetics . 2006 [7]. Mark Stapleton et al. A Drosophila full-length cDNA resource. Genome Biology. 2002 [8]. Li W-H. Molecular Evolution. Sinaur Associates, Sunderland, Massachusetts, 1997. [9]. Mia T. L et al. Novel genes derived from noncoding DNA in Drosophila melanogaster are frequently X-linked and exhibit testis-biased expression. PNAS. 2006 [10]. Wen Wang et al. Origin of sphinx, a young chimeric RNA gene in Drosophila. PNAS.2002 [11]. Koen J.T et al. EMERGING TECHNOLOGIES FOR GENE MANIPULATION IN DROSOPHILA MELANOGASTER. Nature genetics. 2005 [12]. Jolanta Szulc et al. A versatile tool for conditional gene expression and knockdow. Nature methods. 2006 [13]. Stephen, T. T et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nature genetics. 2004 [14]. Hugo J. Bellen et al. The BDGP Gene Disruption Project: Single Transposon Insertions Associated With 40% of Drosophila Genes. Genetics Society of America. 2004 [15]. Personal communication with Zhan. [16]. Zhou, Qi et al. Comparative genomic analysis links karyotypic evolution with genomic evolution in the Indian Muntjac. Chromosoma. 2006 14