Chem 3125 - St. Edwards University
advertisement

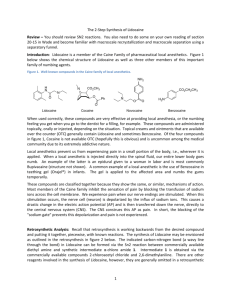
2-15-13 Chem 3125 Experiment 3 The Synthesis of Lidocaine Overall Synthesis: O NH2 HN O Cl O N HN Part B Part A HN Part C N H HSO4 Part A Preparation of -chloro-2,6-dimethylacetanilide O NH2 O Cl NaOAc HN Cl Cl AcOH CAUTION: Handle both the reactant and product with care as they are hazardous materials. USE GLOVES AND WORK IN THE HOOD. Procedure: Week 1 Combine 3.7 mL of 2,6-dimethylaniline with 25 mL of glacial acetic acid (25 mL) and 2.6 mL of α-chloroacetyl chloride (in that order) in a 125-mL Erlenmeyer flask. After warming the solution to 40-50 ˚C using a hot plate, remove the flask from the heat and add the reaction mixture to a 125-mL Erlenmeyer flask containing a solution of 4.90 g sodium acetate trihydrate in 100 mL water. Cool the resulting mixture in an ice/water bath and collect the product by vacuum filtration. Rinse the filter cake with water until the odor of acetic acid can no longer be detected, and dry it as completely as possible by pressing on it with a spatula while the vacuum source is still attached. Transfer the solid to a fresh sheet of filter paper on a watch glass and allow it to air-dry for at least 24 hr. Record the mass of the isolated product. Notes: -chloro-2,6-dimethylacetanilide mp = 145-146 ˚C, MW =197.5 g/mol Required characterization: melting point, IR, NMR (submit ~ 0.10 g) 2-15-13 Part B O O HN Cl HN N HN CAUTION: Handle both the reactants and product with care as they are hazardous materials. USE GLOVES AND WORK IN THE HOOD. Procedure: Week 2 Note- All reagents and equipment used in the first paragraph of the procedure must be dry. Place the α-chloro-2,6-dimethylacetanilide from Part A in a 100-mL round-bottomed flask and add toluene (6.6 mL/gram acetanilide) followed by diethylamine (1.5 mL/gram acetanilide) and a stir bar. Attach a condenser and bring the reaction mixture to a vigorous reflux. After 60 min, allow the mixture to cool to room temp, and isolate the crystalline solid that forms by vacuum filtration. Transfer the filtrate to a separatory funnel and extract it twice with 25-mL portions of 3 M HCl. Combine the acidic aqueous extracts in a 125-mL Erlenmeyer flask and add enough 8M NaOH to make the mixture strongly basic (use pH paper). You should anticipate seeing a thin, dark-yellow oil layer in the flask. Cool the alkaline mixture thoroughly by immersion into an ice/water bath. Once chilled, agitation by vigorous swirling or stirring should aid crystallization of lidocaine; scratching the glass of the flask at the liquid/air interface with a stirring rod may also help. Once crystals have started to form, label and store your flask until the next lab period.*. Week 3 Collect the crude lidocaine by vacuum filtration and wash the solids with water. Determine the mass of the lidocaine and set aside a sample (~0.10 g) for NMR and mp analysis and allow it to dry on a watchglass. Transfer the remaining lidocaine to a 125 mL Erlenmeyer flask for the next step in the process. Notes: Lidocaine MW = 234.3 g/mol, MP = 68 ˚C (a) Stop at the asterisk and allow the lidocaine to crystallize over the next week (b) Required characterization: melting point, NMR 2-15-13 Part C Preparation of lidocaine bisulfate O HN O N HN Part D N H HSO4 Procedure: Crude lidocaine is converted to the crystalline salt, lidocaine bisulfate, by first dissolving it in MTBE (10 mL of solvent/g of lidocaine) and then adding H2SO4 (2 mL/g of lidocaine, 2.2 M in ethanol). Mix the solutions thoroughly and scratch the glass at the air-liquid interface to induce crystallization. Mixing is best accomplished using a magnetic stirrer. The reaction is occurring at the interface between the two layers. Stirring increases the conversion. Dilute the mixture with an equal volume of reagent grade acetone to facilitate filtration, and isolate the precipitated salt by vacuum filtration. Rinse the filter cake with a few mL of cold acetone and air dry the product. Notes: Lidocaine bisulfate: MW = 347.2 g/mol; MP = 210-212 ˚C (a) (b) The product may be recrystallized from a hot solution of water (~ 5 mL) and acetone (100 mL) Required characterization: melting point