Calculate the molar enthalpy of solution
advertisement
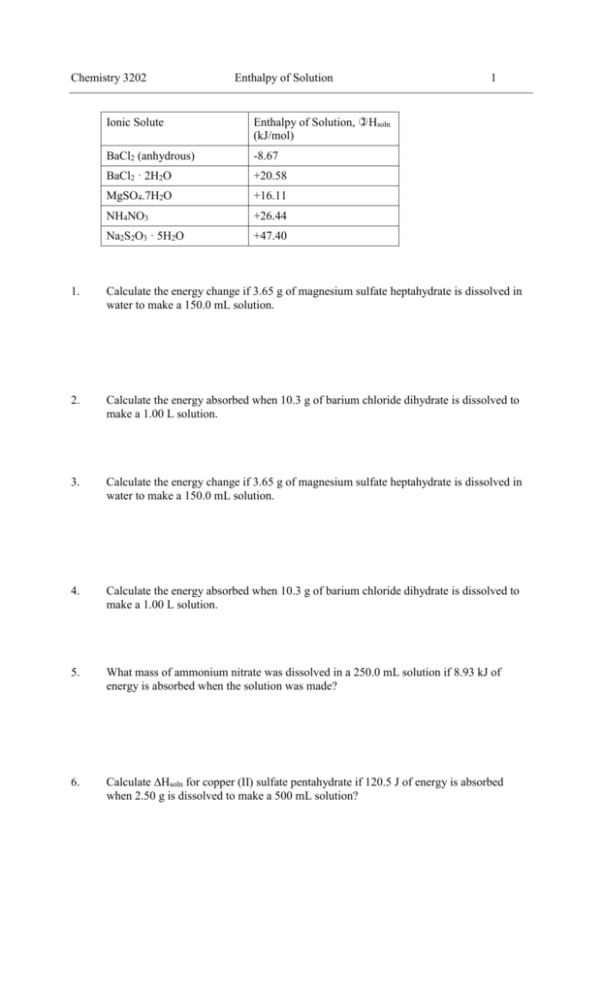
Chemistry 3202 Enthalpy of Solution Ionic Solute Enthalpy of Solution, Hsoln (kJ/mol) BaCl2 (anhydrous) -8.67 BaCl2 · 2H2O +20.58 MgSO4.7H2O +16.11 NH4NO3 +26.44 Na2S2O3 · 5H2O +47.40 1 1. Calculate the energy change if 3.65 g of magnesium sulfate heptahydrate is dissolved in water to make a 150.0 mL solution. 2. Calculate the energy absorbed when 10.3 g of barium chloride dihydrate is dissolved to make a 1.00 L solution. 3. Calculate the energy change if 3.65 g of magnesium sulfate heptahydrate is dissolved in water to make a 150.0 mL solution. 4. Calculate the energy absorbed when 10.3 g of barium chloride dihydrate is dissolved to make a 1.00 L solution. 5. What mass of ammonium nitrate was dissolved in a 250.0 mL solution if 8.93 kJ of energy is absorbed when the solution was made? 6. Calculate Hsoln for copper (II) sulfate pentahydrate if 120.5 J of energy is absorbed when 2.50 g is dissolved to make a 500 mL solution? Chemistry 3202 Enthalpy of Solution 2 7. 5.23 g of sodium thiosulphate pentahydrate is dissolved in 500.0 mL of water. If the initial temperature of the water was 22.8C, what will be the final temperature of the solution? State two assumptions you make in answering this question. 8. When sulphuric acid dissolves in water, a great deal of heat is given off. To measure it, 175 g of water was placed in a coffee-cup calorimeter and chilled to 10oC. Then 49.0 g of pure sulphuric acid, also at 10.0oC was added, and the mixture was quickly stirred with a thermometer. The temperature rose rapidly to 14.9oC. a. Calculate q for the formation of this solution. b. 9. Calculate the molar enthalpy of solution Hsoln in kilojoules per mole of H2SO4. Calculate the mass of anhydrous barium chloride dissolved in a 250.0 mL solution if, when added, the temperature of the mixture increased 3.79C. Chemistry 3202 1. Enthalpy of Solution 3 Upon dissolving two different ionic solutes, one solution gets very warm, while the second gets very cold. Explain how the same process can be exothermic sometimes and endothermic other times. Be specific in your answer - describe all the processes involved and whether they absorb or release energy. Draw a diagram to illustrate your answer. See notes 2. From the data table above, pick an ionic solute that a. has a lattice energy greater than its hydration energy: BaCl22H2O, MgSO47H2O, NH4NO3, Na2S2O35H2O b. has a lattice energy less than its hydration energy. BaCl2(anhydrous) 3. Calculate the energy change if 3.65 g of magnesium sulfate heptahydrate is dissolved in water to make a 150.0 mL solution. q nH so ln 4. Calculate the energy absorbed when 10.3 g of barium chloride dihydrate is dissolved to make a 1.00 L solution. q nH so ln 5. m 3.65 g H so ln (16.11kJ / mol) 0.2385kJ 239 J M 246.52 g / mol m 10.3g H so ln (20.58kJ / mol ) 0.8678kJ 868 J M 244.27 g / mol A student is unsure if a bottle marked "Barium Chloride" is in anhydrous or hydrated form. What simple test could be performed to find out? Put the chemical in water. If the solution warms as it dissolves, it is the anhydrous form since H is negative. If the solution cools as it dissolves, it is the hydrated form, since H is positve. 6. What mass of ammonium nitrate was dissolved in a 250.0 mL solution if 8.93 kJ of energy is absorbed when the solution was made? m M m M H . Solve for m : q H M H M H Mq 80.08 g / mol 8.93J m 27.0 g H 26.44kJ / mol q nH 7. Calculate Hsoln for copper (II) sulfate pentahydrate if 120.5 J of energy is absorbed when 2.50 g is dissolved to make a 500 mL solution? m M m M H Solve for H : q H M m M m Mq (249.72 g / mol )(120.5 J ) H 12036.5 J / mol 12.04kJ / mol m 2.50 g q nH 8. 5.23 g of sodium thiosulphate pentahydrate is dissolved in 500.0 mL of water. If the initial temperature of the water was 22.8C, what will be the final temperature of the solution? State two assumptions you make in answering this question. In this question, we are given information and are asked about the surroundings, as well as being given information about the system (dissolving). Chemistry 3202 Enthalpy of Solution 4 Therefore all of the energy changes add up to zero (First law of thermodynamics or the Law of conservation of energy): qdissolving + qsurroundings = 0 nH + mcT = 0 First assumption: The system is isolated, and all energy changes occur in the solution. No energy changes occur between the solution and the beaker or cup or air. Second assumption: The whole mixture undergoes a temperature change. Since the mixture is mostly composed of water, we will assume the specific heat of the solution is equal to the specific heat of water. 5.23 (47.40kJ / mol ) (505.2 g )( 4.18 J / g C )T 0 248.22 g / mol 0.999kJ (2114 J / C )T 0 (2114 J / C )T 0.999kJ 999 J T 0.473 C 2114 J / C T f Ti T 22.8 C 0.473 C 22.3 C 9. 10. When sulphuric acid dissolves in water, a great deal of heat is given off. To measure it, 175 g of water was placed in a coffee-cup calorimeter and chilled to 10oC. Then 49.0 g of pure sulphuric acid, also at 10.0oC was added, and the mixture was quickly stirred with a thermometer. The temperature rose rapidly to 14.9oC. a. Calculate q for the formation of this solution. Again, we are given information about both the system and surroundings. Therefore this is a calorimetry problem, and we must use the first law of thermodynamics. qsulfuric acid dissolving + qsurroundings = 0 qsulfuric acid dissolving = - qsurroundings qsulfuric acid dissolving = - mcT = -(175 + 49.0)g(4.184J/gC)(4.9C) = -4592J = -4.60kJ b. Calculate the molar enthalpy of solution Hsoln in kilojoules per mole of H2SO4. qsulfuric acid dissolving = -4.60kJ = nH m 49.0 g 4.60kJ H (H ) M 98.09 g / mol 98.09 g / mol H 4.60kJ 9.21kJ / mol 49.0 g Calculate the mass of anhydrous barium chloride dissolved in a 250.0 mL solution if, when added, the temperature of the mixture increased 3.79C. Again the focus is on both system and the surroundings. Must use First law of thermodynamics: qbarium chloride dissolving + qsurroundings = 0 qbarium chloride dissolving = - qsurroundings nH = - mcT m BaCl2 (H ) (m BaCl2 m H 2O )( 4.184 J / g C )(3.79 C ) M m BaCl2 (8.67kJ / mol ) (m BaCl2 250.0 g )(15.86 J / g ) 208.23g / mol (0.0416kJ / g )m BaCl2 m BaCl2 (15.86 J / g ) 3964 J (41.6 J / g )m BaCl2 (15.86 J / g )m BaCl2 3964 J (25.74 J / g )m BaCl2 3964 J m BaCl2 3964 J 154 g 25.74 J / g






