Geological Time - Doral Academy Preparatory
advertisement

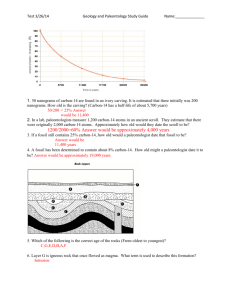
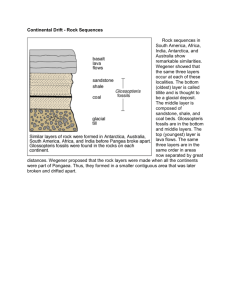
Geological Time The Earth is approximately 4.6 billion years old. Scientists have divided this geological time scale into eons, eras, periods, and epochs. Eons represent the greatest time. Eons are divided into eras. Each era is subdivided into periods. Finally periods are divided into still smaller units called epochs. Below is the Geological Time Scale. The geologic time scale is a visual record or Earth’s history, with the individual units based on changes in the rocks and fossils. Notice that the geological time scale before 650 million years doesn’t have epochs but has a super eon called the Precambrian which includes 3 eons. This is because Precambrian time, there were fewer life forms. These life forms are more difficult to identify and the rocks have been disturbed often. Measuring The Age Of Earth And The Events That Shaped It How understanding the principles of rock layers help to determine the age of rocks. In geology the principal laws are: Principle of Uniformitarianism: Processes that are happening today also happened in the past__________________. Principle of Superposition – The rock on the bottom are older than the rock on the top Principle of Cross-Cutting Relations: A rock is __________ younger than any rock it cuts across. In the above picture a vertical column of magma called a dike cooled into igneous rock. The magma that cooled into igneous rock that cut across the other three layers of rock is younger than the three other layers. Looking at the picture to the right arrange rock layers A, B, C, E, F, G, H, J, K in order from the oldest to the youngest. Ignore letters D and I. Rock layers from the oldest to the youngest: _J,K,F,H,B,G,E,C,A________________________________ Rock layer D (red) going into layers A and B is younger than A and B but older then ____C_, ___F___, ___G and ____H___. Rock layer D represents a pocket of magma that traveled up from beneath layers A and B which hardened into igneous rock. Order the rock layers in order from the oldest to youngest. _________________________________________________ _________________________________________________ __________________________________ Unconformity: Is a place where rock layers are missing. After a rock layer is formed, the area can be uplifted to the Earth’s surface (uplifting can be caused by earthquakes and plate tectonics). After, the process of weathering and erosion can wear the rock layer away. Rock layers often occur above the unconformity, but they are not the kind of rock that would have formed in the same way as the rock layer beneath the unconformity. Example: Order the rock layers in order from the oldest to youngest. _______________________________________________ Order the rock layers in order from the oldest to youngest. _______________________________________________ Two Types of Dating Relative Age Dating: Is a way to describe the age of one event compared to another object or event. Relative age dating is based on comparisons of the age of objects. Thus, this method of dating objects always includes words such as before, after, earlier, later, older, and younger. Example using the principal of superposition: If you found an arrow head in one sedimentary layer of rock and a saber tooth tiger skull in a lower layer of rock, you can use the law of superposition to conclude that the skull is older than the arrow head. Absolute Age: This describes the actual age of an object or event. The absolute ages of objects from long ago are found by analyzing the chemicals in the object or the rock layers in which they were found. Radiometric Dating – This is the most accurate form of dating. This method measures the decay (decrease in size and/or energy) of naturally occurring radioactive isotopes. Example: Carbon-14 Dating (The 14 is the total number of protons and neutrons in the nucleus of the atom) Most atoms of carbon have 6 protons and 6 neutrons in the nucleus of atom. A very few carbon atoms have a different number of neutrons and are called isotopes. In conclusion, an isotope is an atom of the same element with a different amount of neutrons in the nucleus. Carbon-14 is an isotope of carbon and is radioactive. Radioactive describes an element that gives off tiny particles and energy from inside its atom. This means that it tends to give off particles from its nucleus. Above picture show the decay of cacbon-14 to nitrogen-14. The antineutrino will destroy itself and give off energy. The carbon-14 atom changes into an atom of nitrogen-14, which is not radioactive but stable. This change is called radioactive decay. Only about 1 in a 1,000,000,000,000 (trillion) carbon atoms are carbon-14, the majority of the atoms are carbon-12. The dating process compares the ratio of carbon-14 to carbon-12 in an object. All living things contain very small amounts of carbon-14. This carbon is recycled as we live our lives (carbon in food is released as carbon dioxide as we burn food for energy). The amount of carbon-14 remains about the same as we live our lives. When an organism dies no more carbon-14 is obtained and the carbon-14 decays to the stable element nitrogen14. As the carbon-14 decays, the ratio of carbon-14 to carbon-12 in the organic object decreases at a steady rate because the amount of carbon-14 decreases. Scientists have figured out that it takes about 5730 years for half of the carbon-14 to change to nitrogen-14. Picture of how carbon-14 is renewed or produced on the Earth. Another important radioactive isotope is Uranium which decays into the stable element lead. This method has let scientists to determine the approximate age of the Earth as 4.6 billion years old. Below are 3 common radioactive isotopes that are used to date objects. The parent or mother isotope is the radioactive unstable isotope and the daughter isotope is the stable element that forms from decay.