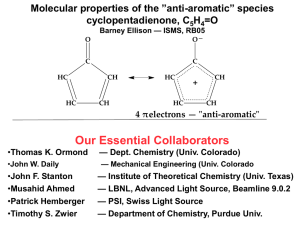
Selected Characterization date for :
advertisement

Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 SUPPORTING INFORMATION AVAILABLE FOR Molecular Batteries: Dendritic FeI Electron-Reservoir Complexes and Reduction of C60 Jaime Ruiz, Charlotte Pradet, François Varret,§ Didier Astruc* Synthetic procedures and selected characterization data for the compounds 4, 5: 4: A solution of [Fe(C5H4CO2H)(C6Me6)][PF6], 2, (0.90 g, 1.9 mmol) in SOCl2 (20mL) was stirred at reflux overnight, then the solution of the acid chloride 3 was allowed to cool down to room temperature, and the solvent was removed under vacuum. CH2Cl2 (50 mL) was added to this red powder of 3, the solution was cooled to 0 °C, and 0.5mL of dry propylamine was slowly added. After 2 hrs. at room temperature, the solvent was removed under vacuum. The residue was disolved in nitromethane and washed with a dilute HPF6 solution and twice with water, then dried over sodium sulfate. The solution was concentrated to 5 mL under reduced pressured, and the solid was precipitated with 70 mL of diethylether, giving a 81% yield of the amide 4 (0.729 g, 1.54 mmol) as a yellow-orange powder. 1 H NMR (CD3CN ppm .) : 6.95 (NH) ; 4.75 (m. 4H, C5H4) ; 3.26 (q, 2H, NHCH2) ; 2.37 (s, 18H, CH3) ; 1.59 (m. 2H, CH2-CH3) ; 0.95 (CH2-CH3). 13C NMR (CD3CN ppm .) : 164.82 (CO) ; 100.46 (Cq, C6Me6) ; 85.05 (Cq, C5H4) ; 80.93 ;77.13 (CH, C5H4) ; 42.51 (NHCH2) ; 23.47 (CH2CH2CH3) ; 17.26 (CH3) ; 11.94 (CH2CH3). IR (Nujol cm-1)C= O=C-NH) ; 840 (PF6). Anal. Calcd. for C21H30NOFePF6 : C, 49.14 ; H, 5.89 ; N, 2.73 . Found: C, 49.13; H, 5.90; N, 3.15. E1/2 (V vs. FeCp2; DMF. 20°C ). –1.850 V. Na/Hg reduction of 4 to 5: A 0.040 g (0.099 mmol) of [FeCp(C6Me6)][PF6], 1[PF6], in 10 mL of THF was stirred with 4.6 g of Na/Hg amalgam (1%, 2 mmol) for 1 h under N2. Then, THF was removed under vacuum, the deep blue-green residue of 1 was extracted from pentane (15mL), and this deep blue-green used was stable for several hours and futher used for characterization (EPR at 77K: gy = 2.067, gx = 2, 000, gz = 1. 920) and further tests. 6: The DAB-dendr-(NH2)64 (0.080 g, 0.0112 mmol) and triethylamine (0.156 mL, 1.07 mmol) were dissolved in dry CH2Cl2 (20 mL). A solution of 3 (0.500g, 1.07 mmol) in CH3CN (40mL) was added dropwise to this mixture. The solution was stirred at room temperature 1 overnight, then the solvent was removed under vacuum, the solid residue was washed twice with saturated sodium carbonate solution and twice with water, dissolved in CH3CN and dried over sodium sulfate. After filtration, the volume was reduced to 5mL, and precipitation from this solution with 50 mL of dry CH2Cl2 yielded the dendrimer 6 (25% , 0.101 g) as a dark brown powder. H NMR (CD3CN ppm .) : 7.69 (br, 64H, NH) ; 4.9 (br. 128H, C5H4) ; 4.6 (br. 128H, C5H4) ; 3.3 (br. 124H, NHCH2) ; 2.3(br. 1152H, CH3 and 372H CH2HNCH2) ; 1.69and 1.49 (br. 252H CH2CH2N). 13C NMR (CD3CN ppm .) : 162.7(C=O) ; 99.2 ( Cq, C6Me6) ; 83.6 (Cq, C5H4) ; 79.6 ; 75.7 (CH, C5H4) ; 51.4 (CH2NCH2 ) ; 38.2 (NHCH2) ; 26.5 (CH2CH2CH3) ; 15.9 (CH3) . IR (Nujol cm-1)C= O=C-NH) ; 840 (PF6). Anal. Calcd. for C1528H2224NOFe64P64F384 : C, 50.65; H, 6.17;. Found : C, 48.73, H, 6.23 E1/2 (V vs FeCp2; DMF. 20°C): –1.850 V. 1 Reaction between C60 and 7. A 0.040 g (0.099 mmol) of [FeCp(C6Me6)][PF6], 1[PF6], in 10 mL of THF was stirred with 4.6 g of Na/Hg amalgam (1%, 2 mmol) for 1 h under N 2. Then, THF was removed under vacuum, the residue of 1 was extracted from pentane (15mL), and was used for the next reaction. The forest-green solution of [FeICpC6Me6], 1, in pentane is slowly added to a CH3CN (15mL) solution of 7 (0.050g, 0.0014 mmol) upon stirring at –40 °C until thesolution turned from blue-green (EPR of 7, see main text and Figure 1) to forest green (equivalence point) for titration. A solution of 7 at –40 °C was slowly added to a toluene solution (30mL) of C60 (0.063g, 0.088mmol) at –40 °C, which immediately provoked the formation of a precipitate, until the blue-green color persisted in solution (equivalence point of the titration). The solvent was removed under vacuum, and the residue was washed twice with CH3CN (the yellow extracts contain 1[PF6]) and with toluene (colorless extract). The black powder of 8 was dried under vacuum and was kept under N2 (0.091g. 0.00127 mmol, 91% yield). The organic solvent resulting from the washing was removed under vacuum and 0.032g (0.079 mmol, 80%) of [FeCp(C6Me6)][PF6] was recovered. EPR spectrum at 298K(see Figure 2) : g = 2.00093; H = 3.12 G Mössbauer spectrum at 77K (see Figure 2): SQ = 1.97 mm/s; IS = 0.56 mm/s (vs. Fe). Captions to the figures on the following pages: 1) Infra-red spectrum of the dendric amide 6 2) 13C NMR spectrum of the dendritic amide 6 3) 1H NMR spectrum of the dendritic amine 6 2 70.39 IR of 6 in Nujol T% 3422 1649 1532 32.66 840 3 13 C NMR of 6 in CD3CN CN HMB HMB C5H4 CH2NCH2 C=O NHCH2 CH2CH2CH2 4 1 H NMR of 6 in CD3CN 5