2. genetic Research objectives
advertisement

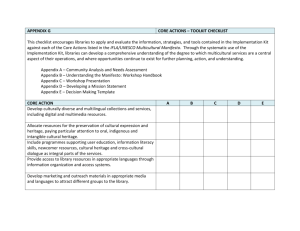
Clinical Study Protocol Appendix D <<1>> Drug Substance <<2>> Study Code <<3>> Appendix Edition Number <<4>> Appendix Date <<Day Month Year>> Appendix D Pharmacogenetics Research NOTE: The authoring instruction box should be deleted from the document before it is finalised To the Author: • The standard text (mandatory) provided in the template is to be used unless stated otherwise. • The dates of the different appendices do not need to be the same as long as they are dated before the final Clinical Study Protocol or at the same day as the final Clinical Study Protocol. • Where Pharmacogenetic (PGx) analysis is a co-primary objective within a clinical study, or where genetic sample collection is a mandatory part of the study, the information relating to PGx research should be captured in the main Clinical Study Protocol template. • Where PGx is a secondary or an exploratory objective for the study the information may be captured within the main Clinical Study Protocol or it may be used as an appendix. • Where the study will be recruiting in territories that are known to have specific issues regarding local regulations for genetics research or large multicentre studies a PGx appendix should be used, where possible. • The main Clinical Study Protocol template contains information relating to the collection of Pharmacogenetics (PGx) samples. If a study is planning to use a PGx appendix ensure that duplicate text in the main Clinical Study Protocol is removed and that reference is, instead, made to the PGx appendix. Clinical Study Protocol (CSP) Appendix D Pharmacogenetics Research 20101020 Clinical Study Protocol Appendix D <<1>> Drug Substance <<2>> Study Code <<3>> Appendix Edition Number<<4>> Appendix Date <<Day Month Year>> Clinical Study Protocol (CSP) Appendix D Pharmacogenetics Research 20101020 22 Clinical Study Protocol Appendix D <<1>> Drug Substance <<2>> Study Code <<3>> Appendix Edition Number<<4>> Appendix Date <<Day Month Year>> TABLE OF CONTENTS PAGE TITLE PAGE ................................................................................................. 1 TABLE OF CONTENTS .................................................................................... 3 LIST OF ABBREVIATIONS AND DEFINITION OF TERMS ...................................... 4 1. BACKGROUND AND RATIONALE ....................................................... 5 2. GENETIC RESEARCH OBJECTIVES ..................................................... 5 3. GENETIC RESEARCH PLAN AND PROCEDURES .................................... 6 3.1 3.1.1 3.1.2 3.1.3 3.1.4 Selection of genetic research population ............................................ 6 Study selection record ..................................................................... 6 Inclusion criteria ............................................................................. 6 Exclusion criteria ............................................................................ 6 Discontinuation of subjects from this genetic research......................... 6 3.2 Collection of samples for genetic research ......................................... 7 3.3 Coding and storage of DNA samples .................................................. 7 4. ETHICAL AND REGULATORY REQUIREMENTS ..................................... 8 4.1 Informed consent ........................................................................... 8 4.2 Subject data protection ................................................................... 9 5. DATA MANAGEMENT ....................................................................... 9 6. STATISTICAL METHODS AND DETERMINATION OF SAMPLE SIZE ........ 10 7. LIST OF REFERENCES ................................................................... 10 Clinical Study Protocol (CSP) Appendix D Pharmacogenetics Research 20101020 33 Clinical Study Protocol Appendix D <<1>> Drug Substance <<2>> Study Code <<3>> Appendix Edition Number<<4>> Appendix Date <<Day Month Year>> LIST OF ABBREVIATIONS AND DEFINITION OF TERMS Abbreviation or Explanation special term CSR Clinical Study Report DNA Deoxyribonucleic acid LIMS Laboratory information management system PGx Pharmacogenetics Clinical Study Protocol (CSP) Appendix D Pharmacogenetics Research 20101020 44 Clinical Study Protocol Appendix D <<1>> Drug Substance <<2>> Study Code <<3>> Appendix Edition Number<<4>> Appendix Date <<Day Month Year>> 1. BACKGROUND AND RATIONALE To the Author: Briefly justify the reasons for the inclusion of the genetic component of the study explaining the strategy and hypothesis on which this is based and how it fits into the clinical development plan. <<Insert Sponsor’s Name>> intends to perform genetic research in the <<drug name>> clinical development programme to explore how genetic variations may affect the clinical parameters associated with <<drug name and/or agents used in combination or as comparators>>. Collection of DNA samples from populations with well described clinical characteristics may lead to improvements in the design and interpretation of clinical trials and, possibly, to genetically guided treatment strategies. <<Future research may suggest other genes or gene categories as candidates for influencing not only response to <<drug name and/or agents used in combination or as comparators >> but also susceptibility to << (use MedDRA terminology when possible: the ‘response’/disease>> for which <<drug name>> may be evaluated. Thus, this genetic research may involve study of additional un-named genes or gene categories, but only as related to <<disease susceptibility and drug action.>> OR <<It is emphasised that the Sponsor will only look for markers within genes relevant to the mode of action of, and response to << drug name and/or agents used in combination or as comparators >>, and <<disease/response>> under study within the current Clinical Study Protocol. No other research will be performed on the samples.>> 2. GENETIC RESEARCH OBJECTIVES To the Author: • Use the default paragraph below. • Where the study team has agreed that an objective with a “narrow” scope of research is appropriate, the above paragraph should be edited to provide the specific details– eg, research only on drug response, or only on named genes etc. • Consult the local or therapeutic area pharmacogenetics advisor/ project for advice. The objective of this research is to collect and store DNA for future exploratory research into genes/genetic variation that may influence response (ie, distribution, safety, tolerability and efficacy) and/or <<susceptibility to disease>> << (use MedDRA terminology when possible: the ‘response’/disease>> to XXXX <<and/or agents used in combination and/or as comparators>>. Clinical Study Protocol (CSP) Appendix D Pharmacogenetics Research 20101020 55 Clinical Study Protocol Appendix D <<1>> Drug Substance <<2>> Study Code <<3>> Appendix Edition Number<<4>> Appendix Date <<Day Month Year>> 3. GENETIC RESEARCH PLAN AND PROCEDURES 3.1 Selection of genetic research population 3.1.1 Study selection record <<All or other criteria>> subjects will be asked to participate in this genetic research. Participation is voluntary and if a subject declines to participate there will be no penalty or loss of benefit. The subject will not be excluded from any aspect of the main study. 3.1.2 Inclusion criteria For inclusion in this genetic research, subjects must fulfil all of the inclusion criteria described in the main body of the Clinical Study Protocol and: • Provide informed consent for the genetic sampling and analyses. 3.1.3 Exclusion criteria Exclusion from this genetic research may be for any of the exclusion criteria specified in the main study or any of the following: To the Author: The PGx exclusion criteria below apply to blood and saliva samples. If a buccal swab is being taken for DNA extraction and analysis the two exclusion criteria listed below should be deleted. • <<Previous allogeneic bone marrow transplant>> • <<Non-leukocyte depleted whole blood transfusion in 120 days of genetic sample collection>> 3.1.4 Discontinuation of subjects from this genetic research Specific reasons for discontinuing a subject from this genetic research are: Withdrawal of consent for genetic research: Subjects may withdraw from this genetic research at any time, independent of any decision concerning participation in other aspects of the main study. Voluntary discontinuation will not prejudice further treatment. Procedures for discontinuation are outlined in Section 7.5 of the main Clinical Study Protocol. Clinical Study Protocol (CSP) Appendix D Pharmacogenetics Research 20101020 66 Clinical Study Protocol Appendix D <<1>> Drug Substance <<2>> Study Code <<3>> Appendix Edition Number<<4>> Appendix Date <<Day Month Year>> 3.2 Collection of samples for genetic research To the Author: • Describe when samples will be collected. • A blood sample is the preferred sample type for the majority of genetic research samples. • Capture details of sampling, handling, storage and transport of the samples in the Laboratory Manual. Samples may be collected at any time after randomisation, however; ideally samples should be collected early in the study to avoid bias in the sample collection (ie, failure to collect samples from patients who have suffered an AE etc). The blood <<or other appropriate sample type eg, saliva or buccal swab>> sample for genetic research will be obtained from the subjects at Visit << X at or after>>randomisation. Although genotype is a stable parameter, early sample collection is preferred to avoid introducing bias through excluding patients who may withdraw due to an adverse event (AE), such patients would be important to include in any genetic analysis. If for any reason the sample is not drawn at Visit <<X>>, it may be taken at any visit until the last study visit. Only one sample should be collected per subject for genetics during the study. Samples will be collected, labelled, stored and shipped as detailed in the Laboratory Manual. For blood volume, see Section 7.1 of the Clinical Study Protocol. 3.3 Coding and storage of DNA samples To the Author: The recommended default storage time is 25 years; this may vary in different territories. In some circumstances, there may be a rationale for the storage of DNA samples until they are depleted. When defining the storage time local laws and regulations for the storage of genetic samples must be considered. The processes adopted for the coding and storage of samples for genetic analysis are important to maintain subject confidentiality. Samples will be stored for a maximum of <<XX years>>, from the date of last subject last visit, after which they will be destroyed. DNA is a finite resource that is used up during analyses. Samples will be stored and used until no further analyses are possible or the maximum storage time has been reached. For all samples irrespective of the type of coding used the DNA will be extracted from the blood <<or other appropriate sample type eg, saliva or buccal swab>> sample. The DNA sample will be assigned a unique number replacing the information on the sample tube. Thereafter, the DNA sample will be identifiable by the unique DNA number only. The DNA number is used to identify the sample and corresponding data at the Sponsor’s genetics laboratories, or at the designated contract laboratory. No personal details identifying the individual will be available to any person (Sponsor’s employee or contract laboratory staff working with the DNA.) Clinical Study Protocol (CSP) Appendix D Pharmacogenetics Research 20101020 77 Clinical Study Protocol Appendix D <<1>> Drug Substance <<2>> Study Code <<3>> Appendix Edition Number<<4>> Appendix Date <<Day Month Year>> <<Choose one of the following procedures of the coding procedure to be used: Option 1 is the preferred choice Where samples and data will be “single coded” (Option 1): The samples and data for genetic analysis in this study will be single coded. The link between the subject enrolment/randomisation code and the DNA number will be maintained and stored in a secure environment, with restricted access WITHIN the Clinical Genotyping Group Laboratory Information Management System (LIMS) at <<insert Sponsor’s Name>>. The link will be used to identify the relevant DNA samples for analysis, facilitate correlation of genotypic results with clinical data, allow regulatory audit and to trace samples for destruction in the case of withdrawal of consent.>> <<Where samples and data will be “double coded” (Option 2): The samples and data for genetic analysis in this study will be double coded. The link between the subject enrolment/randomisation code and the DNA number will be maintained and stored in a secure environment OUTSIDE of the Clinical Genotyping Group LIMS database at <<insert the Sponsor’s Name>>. The link file will be used to identify the relevant DNA samples for analysis, facilitate correlation of genotypic results with clinical data, allow regulatory audit and to trace samples for destruction in the case of withdrawal of consent.>> 4. ETHICAL AND REGULATORY REQUIREMENTS The principles for ethical and regulatory requirements for the study, including this genetics research component, are outlined in Section 8 of the main Clinical Study Protocol. 4.1 Informed consent To the Author: • Describe when and how the informed consent will be obtained. • Include the following standard text where the informed consent for genetic analyses is separate to the consent for the main study. The genetic component of this study is optional and the subject may participate in other components of the main study without participating in the genetic component. To participate in the genetic component of the study the subject must sign and date both the consent form for the main study and the genetic component of the study. Copies of both signed and dated consent forms must be given to the subject and the original filed at the study centre. The principal investigator(s) is responsible for ensuring that consent is given freely and that the subject understands that they may freely discontinue from the genetic aspect of the study at any time. Clinical Study Protocol (CSP) Appendix D Pharmacogenetics Research 20101020 88 Clinical Study Protocol Appendix D <<1>> Drug Substance <<2>> Study Code <<3>> Appendix Edition Number<<4>> Appendix Date <<Day Month Year>> 4.2 Subject data protection To the Author: Delete duplicate text in the main CLINICAL STUDY PROTOCOL. The Sponsor will not provide individual genotype results to subjects, any insurance company, any employer, their family members, general physician or any other third party, unless required to do so by law. Extra precautions are taken to preserve confidentiality and prevent genetic data being linked to the identity of the subject. In exceptional circumstances, however, certain individuals might see both the genetic data and the personal identifiers of a subject. For example, in the case of a medical emergency, the Sponsor’s Physician or an investigator might know a subject’s identity and also have access to his or her genetic data. Also Regulatory authorities may require access to the relevant files, though the subject’s medical information and the genetic files would remain physically separate. 5. DATA MANAGEMENT To the Author: • Delete this duplicate text from the CLINICAL STUDY PROTOCOL and reference the PGx Appendix. • Indicate whether data will be reported in the CSR for the main study or will be reported separately. • Where genetic data will be merged with the clinical database use the standard text given in option 1 below. • Where genetic data will not be merged with the clinical database use standard text given in option 2 below. Any genotype data generated in this study will be stored in the Sponsor’s genotyping LIMS database, or other appropriate secure system within the Sponsor and/or third party contracted to work with the Sponsor to analyze the samples. The results from this genetic research may be reported in the CSR for the main study, or in a separate report as appropriate. <<Choose one of the following procedures, Option 1: Genotype data will be transferred to the clinical database, and merged with the clinical data from the main study, prior to the statistical analysis and reporting of the study. >> <<or Option 2: Some or all of the clinical datasets from the main study may be merged with the genetic data in a suitable secure environment separate from the clinical database.>> Clinical Study Protocol (CSP) Appendix D Pharmacogenetics Research 20101020 99 Clinical Study Protocol Appendix D <<1>> Drug Substance <<2>> Study Code <<3>> Appendix Edition Number<<4>> Appendix Date <<Day Month Year>> 6. STATISTICAL METHODS AND DETERMINATION OF SAMPLE SIZE STATISTICAL METHODS AND DETERMINATION OF SAMPLE SIZE The number of subjects that will agree to participate in the genetic research is unknown. It is therefore not possible to establish whether sufficient data will be collected to allow a formal statistical evaluation or whether only descriptive statistics will be generated. A statistical analysis plan will be prepared where appropriate. 7. LIST OF REFERENCES <<>> Clinical Study Protocol (CSP) Appendix D Pharmacogenetics Research 20101020 10 10