Photoelectric Effect Lab: PhET Simulation Activity
advertisement

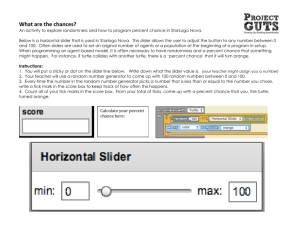
Date Performed: ____________ Date Due: ______________ Date Handed In: ___________ GOOD COPY If this copy is neat, you do not need to rewrite it. If not, rewrite it neatly on loose leaf or a clean copy of this sheet found on the class website. Neatness is part of the grade for the lab. Raw Score: / 68 Final Grade: / 50 IB 12 Name: ____________________ Partner: ACTIVITY: The Photo-Electric Effect Purpose: To understand the effects of frequency, intensity and potential difference on the photoelectric effect apparatus. Go to http://phet.colorado.edu. Click on Browse Sims in the lower right corner. From the left menu select Quantum Phenomena. Click on The Photoelectric Effect (on the right side of your screen). Click on Run Now! to open the simulation. You should see the screen shown at right. General description of the features of the simulation Intensity slider (attached to light source) enables you to change the intensity of the external source of electromagnetic radiation from 0% to 100%. Wavelength slider (attached also to light source) enables you to change the wavelengths of the external radiation from Infrared (IR; very right) to Ultraviolet (UV very left). Target menu (the very top-right side) enables you to change the type of metal (photoemissive surface) from which the electrons are ejected, for example: sodium, zinc, copper, platinum, or calcium. Potential slider (attached to the battery, lower part of the diagram) enables you to change the potential difference between the two metal plates from -8.0V to +8.0V. Note: Red color of the plate indicates a positive terminal, blue indicates a negative terminal. Part One: Investigating Light and Photons In this section you are going to investigate the relationship between light intensity, energy and photons. 1. With the wavelength set at 400 nm, move the intensity slider from 0% to 100% to see what effect this has on the light. (For this part, ignore any effects your actions have on the electrons emitted from the plate.) a) What do you observe happening to the brightness of the light? [1] b) What can you infer is happening to the amount of energy transmitted by the light? [1] 2. Move the intensity slider back to 0%. Choose OPTIONS/SHOW PHOTONS from the toolbar at the upper left. From now on, you should see individual photons instead of a light beam from the light source. Move the intensity slider from 0% to 100% to see what effect this has on the beam of photons. As the intensity increases, what effect, if any, does this have on: a) the number of photons? b) the frequency of the photons? c) the energy of each individual photon? d) the total energy of the light beam? e) Explain your answer to part (d) using your answers to the other parts of this question. [1] [1] [1] [1] [2] 3. Now, set the intensity slider to 50% and vary the wavelength from infrared to ultraviolet. As the slider moves from IR to UV, what effect, if any, does this have on: a) the frequency of the photons? b) the energy of individual photon? c) the total energy of the light beam? d) the number of photons? e) Explain your answer to part (b) using your answers to the other parts of this question. [1] [1] [1] [1] [2] Part Two: Investigating Frequency In this section you are going to investigate the relationship between the frequency of the incident light and the energy of the emitted electrons. 4. Click in the check boxes to display all three graphs. Set the intensity slider to 100%, the voltage slider to 0.00 V, the wavelength to IR, and the target to sodium. Slowly move the wavelength slider to UV and watch the graph of electron energy vs. light frequency. a) Sketch it on the axes at right. Pay careful attention to the x-intercept and slope. [2] b) State the value and significance of the x-intercept. [2] c) Calculate the value of the work function for this metal. [2] d) Pick two points on the linear section of the graph and calculate the slope. Be sure to convert the units to joules. Show your work clearly. [4] e) What is the significance of the slope? f) State and compare your value of the slope to the literature value. [1] [2] 6. Set the wavelength slider at a value for which no electrons are emitted from the plate. Move the [1] intensity slider from 0% to 100%. What effect does this have on the number of electrons ejected? 7. Very carefully find the wavelength for which electrons just begin to be ejected from the plate and leave the wavelength slider at this value. (You can also type in values to set the value more precisely than using the slider.) a) State the value of this wavelength. [1] b) Calculate the frequency associated with this wavelength. Show your work. [2] c) Compare your answer to (b) to the x-intercept of the graph. [1] d) Move the intensity slider from 0% to 100%. What effect does this have on the number of electrons ejected? [1] 8. In order to investigate the kinetic energies of the electrons, set the wavelength slider at a value in the violet region of the EM spectrum and set the intensity slider to 100%. a) Look at the ejected electrons. Notice that they are not all moving at the same speed. Why do [2] some electrons have more kinetic energy than others? b) Describe and explain the difference between the maximum kinetic energy of these electrons and the maximum kinetic energy of the electrons emitted in step 7 when the light was at the threshold wavelength. Include in your answer the concept of the work function. [3] 9. In order to investigate the different types of targets, set the intensity slider to 100% and select a zinc target. Move the wavelength slider from IR to UV to trace out a new graph of energy vs. frequency. a) Carefully sketch it on the axes at right. [2] b) Compare the threshold frequency of zinc to that of sodium. [1] c) Compare the slope of zinc graph to the slope of the sodium graph. [1] Part Three: Investigating Intensity In this section you are going to investigate the relationship between the intensity of the incident light and the current in the circuit. 10. Set the target to sodium, the voltage to 0.00V, and the wavelength to a value in the mid-UV range. Vary the intensity slider to see the relationship between the current in the circuit and the intensity of the light. Sketch the relationship on the axes at right. [2] 11. Explain the relationship you have sketched in terms of the interactions between the photons and the electrons. [2] Part Four: Investigating Potential Difference In this section you are going to investigate the relationship between the potential difference in the circuit and the current in the circuit. 12. Set the target to sodium, the wavelength to approximately 240 nm, and the intensity to 50%. Move the potential slider left and right to vary the voltage of the battery. Sketch the relationship this relationship on the axes at right. [2] 13. The value for the x-intercept is known as the “stopping potential.” a) State the value of the stopping potential for this intensity. b) Suggest a reason for this name. (Hint: Watch the electrons.) [1] [1] 14. Keep the same wavelength but increase the intensity to 75%. a) Again, vary the potential and sketch the relationship on the same set of axes. Label which graph is for 50% and which is for 75% intensity. b) Compare the maximum currents for the two graphs and suggest a reason for the difference. [2] c) Compare the stopping potentials for the two graphs and suggest an explanation. [2] [2] Conclusions: 15. Explain how classical theory described light and how the intensity and frequency of the light affected its energy. 16. Describe one result of this experiment that cannot be explained by the classical theory of light. 17. Explain how Einstein described light and how the intensity and frequency of the light affected its energy. [3] [2] [3] 18. Describe how Einstein’s view of light gives a better explanation than classical theory of the result [2] you chose in question 16. [2] neatness