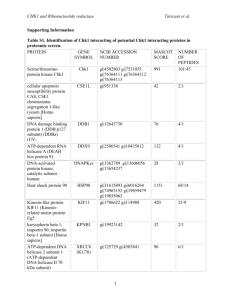
Supplementary Data (doc 4618K)
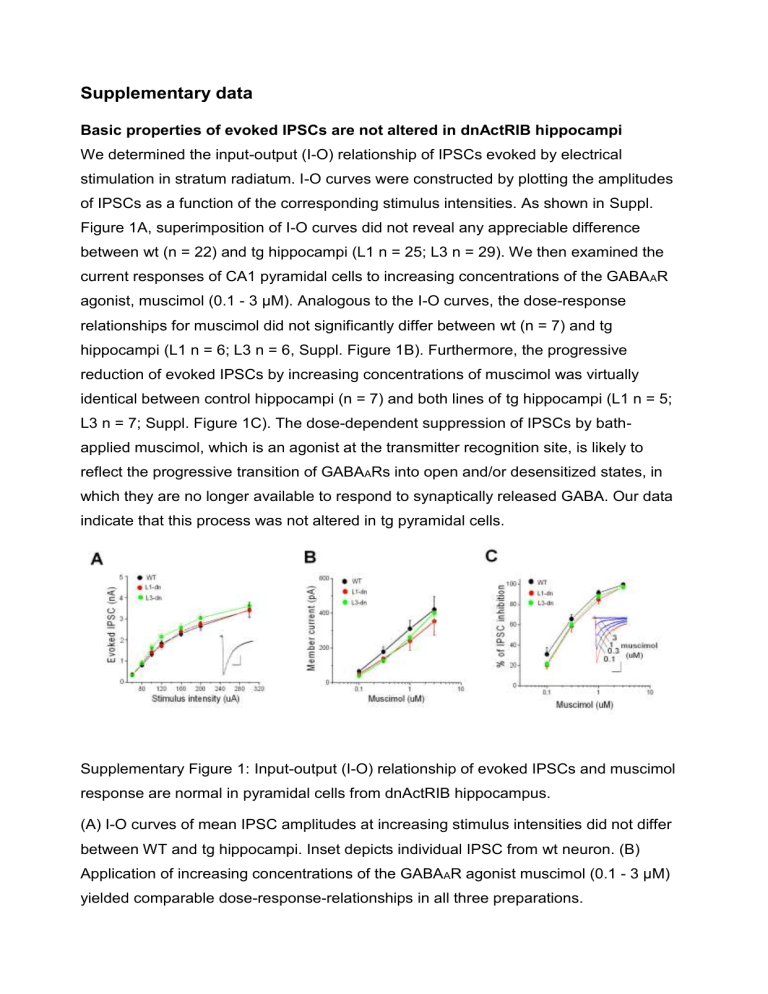
Supplementary data
Basic properties of evoked IPSCs are not altered in dnActRIB hippocampi
We determined the input-output (I-O) relationship of IPSCs evoked by electrical stimulation in stratum radiatum. I-O curves were constructed by plotting the amplitudes of IPSCs as a function of the corresponding stimulus intensities. As shown in Suppl.
Figure 1A, superimposition of I-O curves did not reveal any appreciable difference between wt (n = 22) and tg hippocampi (L1 n = 25; L3 n = 29). We then examined the current responses of CA1 pyramidal cells to increasing concentrations of the GABA
A
R agonist, muscimol (0.1 -
3 µM). Analogous to the I-O curves, the dose-response relationships for muscimol did not significantly differ between wt (n = 7) and tg hippocampi (L1 n = 6; L3 n = 6, Suppl. Figure 1B). Furthermore, the progressive reduction of evoked IPSCs by increasing concentrations of muscimol was virtually identical between control hippocampi (n = 7) and both lines of tg hippocampi (L1 n = 5;
L3 n = 7; Suppl. Figure 1C). The dose-dependent suppression of IPSCs by bathapplied muscimol, which is an agonist at the transmitter recognition site, is likely to reflect the progressive transition of GABA
A
Rs into open and/or desensitized states, in which they are no longer available to respond to synaptically released GABA. Our data indicate that this process was not altered in tg pyramidal cells.
Supplementary Figure 1: Input-output (I-O) relationship of evoked IPSCs and muscimol response are normal in pyramidal cells from dnActRIB hippocampus.
(A) I-O curves of mean IPSC amplitudes at increasing stimulus intensities did not differ between WT and tg hippocampi. Inset depicts individual IPSC from wt neuron. (B)
Application of increasing concentrations of the GABA
A
R agonist muscimol (0.1 - 3 µM) yielded comparable dose-response-relationships in all three preparations.
(C) Increasing decline of evoked IPSC at increasing concentrations of bath-applied muscimol was virtually identical in all three preparations. Inset displays representative recording from tg neuron. Calibration bars in (A,C) were 50 ms and 500 pA.
Immunohistochemistry of GABA
A
Rs and GABA
B
Rs in WT and dnActRIB hippocampi does not reveal gross abnormalities in subunit expression
The distribution of GABA
A
and GABA
B
receptor subunits in the mouse hippocampal formation has been described in several previous studies (Crestani et al., 2002; Peng et al., 2002; Straessle et al., 2003; Wei et al., 2003; Gassmann et al., 2004; Prenosil et al., 2006). Each GABA
A
receptor subunit has a unique distribution pattern, defined by changes in staining intensity across subfields and layers of the hippocampal formation.
GB1b is the predominant GABA
B
receptor isoform in the hippocampus. It forms functional receptors by oligomerization with GB2, and expectedly both subunits have a similar regional distribution, with the highest concentration of stratum lacunosummoleculare of CA1, followed by stratum oriens and pyramidale of CA3 and the dentate gyrus molecular layer.
In the wt mice investigated here, no variation from the reported distribution patterns was observed for either GABA
A receptors, as illustrated for the subunits α1, α2, α3, α4,
α5,
2, and
, or GABA
B
receptors, as shown for GABA
B
-R2 (Suppl. Figure 2). The use of antigen retrieval increases signal to noise by markedly reducing background staining. No change was detectable for any of the markers investigated in dnActRIB mice, affecting either the regional or laminar distribution of GABA
A
or GABA
B
receptor subunits in the hippocampal formation. The staining pattern and intensity were remarkably consistent among animals and it was impossible to distinguish sections of transgenic mice from those of wildtype. Unlike electrophysiology, immunohistochemistry does not distinguish between functional receptors at the cell surface and internalized receptors or even unassembled subunit proteins. However, unlike the function, the overall expression and distribution of GABA receptors is apparently not disturbed in dnActRIB mice.
Supplementary Figure 2. Immunohistochemistry of GABA
A
Rs and GABA
B
Rs in hippocampus of wt and dnActRIB mice
Each pair of photomicrographs depicts transverse sections through the dorsal hippocampus of wildtype (wt) and dnActRIB mice processed for immunoperoxidase staining with antibodies against the GABA
A receptor subunits α1 (A1-A2), α2 (B1-B2),
α3 (C1-C2), α4 (D1-D2), α5 (E1-E2),
2 (F1-F2), and
(G1-G2) and against the
GABA
B
receptor 2 subunit (H1-H2). Each pair of images depicts a representative section from either genotype. No change in regional or laminar distribution is evident for any of the markers depicted. Scale bar, 200 µm.
Experimental procedures
Immunohistochemistry
Adult dnActRIB (line 3) and wt littermates (n=6) were deeply anesthetized by intraperitoneal administration of sodium pentobarbital ( Nembutal; 50 mg/kg; i.p.) and perfused through ascending aorta with 4% paraformaldehyde in 0.15 M phosphate buffer containing 15% of saturated picric acid solution (pH 7.4). Brains were removed, postfixed for 4 hours in the same solution and processed for antigen-retrieval as described (Fritschy et al., 1998). They were cryoprotected in PBS containing 30% sucrose, frozen with powdered dry ice, and cut transversally at a thickness of 40 μm with a sliding microtome. Sections were collected in PBS and stored in an antifreeze solution (15% glucose and 30% ethylene glycol in 50mM phosphate buffer, pH 7.4) prior to use.
Standard immunoperoxidase staining using the Vectastain Elite kit (Vector
Laboratories, Burlingham, CA) was performed (Fritschy et al., 1998) using guinea pig and rabbit antisera against the GABA
A
receptor
1,
2,
3,
4,
5,
, and
2 subunit and against the GABA
B
receptor 1b and 2 subunits (Suppl. Table 1). All antibodies have been used in previous studies under identical conditions and are well characterized for their specificity (Fritschy and Mohler, 1995; Peng et al., 2002;
Fritschy et al., 2004; Kralic et al., 2006). Sections from transgenic and wildtype mice were processed in parallel under identical conditions to minimize variations in labeling intensity.
For analysis, sections were visualized using a bright-field microscope (Zeiss Axioskop,
Jena, Germany). The regional distribution of each subunit, as assessed by the relative staining intensity across brain areas was compared between genotypes, using 4 sections per mouse. In the hippocampal formation, the laminar distribution of each subunit in each subregion (CA1, CA3, dentate gyrus, and subiculum) was analyzed.
For illustration, digital photomicrographs were taken with a color camera. No adjustments were made for contrast or brightness.
Supplementary Table 1: List of primary antibodies
Subunit
GABA
A
receptor
1
Host species
Guinea pig serum dilution
1:20000 source
Home-made subunit subunit
2 Guinea pig, affinity purified
1:1000 Home-made
3 Guinea pig serum 1:4000 Home-made subunit subunit
4 Rabbit, affinity purified
1:1000 Dr. W. Sieghart,
Vienna
5 Guinea pig serum 1:3000 Home-made subunit
subunit Rabbit, 1:2000 affinity purified
2 subunit Guinea pig serum 1:2500
Dr. W. Sieghart,
Vienna
Home-made
GABA
B
-R1b Purified guinea pig
IgG
1:3000
Guinea pig serum 1:3000
Home-made
GABA
B
-R2 Chemicon,
Temecula, CA; No
AB5394
References for supplementary data
Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluethmann H, Mohler H,
Rudolph U (2002) Trace fear conditioning involves hippocampal α5 GABA
A receptors. Proc Natl Acad Sci USA 99:8980-8985.
Fritschy JM, Mohler H (1995) GABA
A
-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp
Neurol 359:154-194.
Fritschy JM, Weinmann O, Wenzel A, Benke D (1998) Synapse-specific localization of
NMDA- and GABA
A
-receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol 390:194-210.
Fritschy JM, Sidler c, Parpan F, Gassmann M, Kaupmann K, Bettler B, Benke D (2004)
Independent maturation of the GABAB receptor subunits GABAB1 and
GABAB2 during postnatal development in rodent brain. The Journal of
Comparative Neurology 477:235-252.
Gassmann M, Shaban H, Vigot R, Sansig G, Haller C, Barbieri S, Humeau Y, Schuler
V, Müller M, Kinzel B, Klebs K, Schmutz M, Froestl W, Heid J, Kelly PJ, Gentry
C, Jaton A, Van der Putten H, Mombereau C, Lecourtier L, Mosbacher J, Cryan
JF, Fritschy JM, Lüthi A, Kaupmann K, Bettler B (2004) Redistribution of
GABA
B(1)
protein and atypical GABA
B
responses in GABA
B(2)
-deficient mice. J
Neurosci 24:6086-6097.
Kralic JE, Sidler C, Parpan F, Homanics G, Morrow AL, Fritschy JM (2006)
Compensatory alteration of inhibitory synaptic circuits in thalamus and cerebellum of GABA
A receptor α1 subunit knockout mice. J Comp Neurol
495:408-421.
Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR
(2002) GABA
A
Altered receptor changes in α subunit-deficient mice: and expression of α4 α2 subunits in the forebrain. J Comp Neurol 446:179-
197.
Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE (2006)
Specific subtypes of GABA
A
receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. May 17; [Epub ahead of print].
Straessle A, Loup F, Arabadzisz D, Ohning GV, Fritschy JM (2003) Rapid and longterm alterations of hippocampal GABA
B
receptors in a mouse model of temporal lobe epilepsy. Eur J Neurosci 18:2213-2226.
Wei W, Zhang N, Peng ZC, Houser CR, Mody I (2003) Perisynaptic localization of δ subunit-containing GABA
A
receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23:10650-10661.