Protein synthesis
advertisement

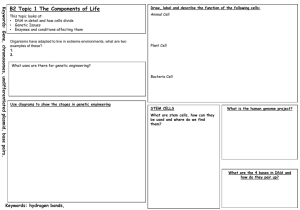
Name: ____________________________ Homework/class-work Unit#4 Protein synthesis, enzymes and mutations (25 points) Think and try every question. There is no reason for a blank response or an I don’t know. Any blanks will receive a zero. Every assignment must be done on a separate piece of paper. Each assignment must be complete, neat, in complete sentences and done on time for full credit. Any assignment may be used as a take home or pop quiz at any time. One missing or late assignment will lose 5 points, 2 will lose 15 points, 3 will be considered incomplete and given a zero. 1. Gene expression reading: Date: __________________ Protein synthesis During the 1950s and 1960s, it became apparent that DNA is essential in the synthesis of proteins. Proteins are used in enzymes and as structural materials in cells. Many specialized proteins function in cellular activities. For example, in humans, the hormone insulin and the muscle cell filaments are composed of protein. The hair, skin and nails of humans are composed of proteins, as are all the hundreds of thousands of enzymes in the body. The key to a protein molecule is how the amino acids are linked. The sequence of amino acids in a protein is a type of code that specifies the protein and distinguishes one protein from another. A genetic code in the DNA determines the amino acid sequence. The genetic code consists of the sequence of nitrogenous bases in the DNA. How the nitrogenous base code is translated to an amino acid sequence in a protein is the basis for protein synthesis. For protein synthesis to occur, several essential materials must be present, such as a supply of the 20 amino acids, which comprise most proteins. Another essential element is a series of enzymes that will function in the process. DNA and another form of nucleic acid called ribonucleic acid (RNA) are essential. RNA is the nucleic acid that carries instructions from the nuclear DNA into the cytoplasm, where protein is synthesized. RNA is similar to DNA, with three exceptions. First, the carbohydrate in RNA is ribose rather than deoxyribose, second, RNA nucleotides contain the pyrimidine uracil rather than thymine and finally DNA is double stranded and RNA is a single strand. Types of RNA In the synthesis of protein, three types of RNA function. The first type called ribosomal RNA (rRNA). This form of RNA is used to manufacture ribosomes. Ribosomes are ultramicroscopic particles of rRNA and protein. They are the places (the chemical “workbenches”) where amino acids are linked to one another to synthesize proteins. Ribosomes may exist along the membrane of the endoplasmic reticulum of in the cytoplasm of the cell. A second important type of RNA is transfer RNA (tRNA). Transfer RNA exists in the cell cytoplasm and carries amino acids to the ribosome for protein synthesis. When protein synthesis is taking place, enzymes link tRNA molecules to amino acids in a highly specific manner. For example, tRNA molecule X will link only to amino acid X; tRNA Y will link only to amino acid Y. The third form of RNA is messenger RNA (mRNA). In the nucleus, messenger RNA receives the genetic code in the DNA and carries the code into the cytoplasm where protein synthesis takes place. Messenger RNA is synthesized in the nucleus at the DNA molecule. During the synthesis, the genetic information is transferred from the DNA molecule to the mRNA molecule. In this way, a genetic code can be used to synthesize a protein in a distant location. Transcription Transcription is one of the first processes in the mechanism of protein synthesis. In transcription, a complementary strand of mRNA is synthesized according to the nitrogenous base code of DNA. To begin, the DNA double helix opens and the enzyme RNA polymerase binds to an area of the DNA strand that serves as a template for making the mRNA strand. The enzyme selects complementary bases from available nucleotides and positions them in an mRNA molecule according to the principle of complementary base pairing. The chain of mRNA lengthens until a “stop” message is received and the mRNA is released. The nucleotides of the DNA strand are read in groups of three. Each group is a codon. Thus, a codon may be CGA, or TTA, or GCT, or any other combination of the four bases, depending on their sequence in the DNA strand. Each codon will later serve as a “code word” for an amino acid. Thus, the mRNA molecule consists of nothing more than a series of codons received from the genetic message in DNA. Translation The genetic code is transferred to an amino acid sequence in a protein through the translation process, which begins with the arrival of the mRNA molecule at the ribosome (rRNA). When the mRNA molecule reaches the ribosome the tRNA molecules were uniting with their specific amino acids. The tRNA molecules then began transporting their amino acids to the ribosomes to meet the mRNA molecule. After it arrives at the ribosome, the mRNA molecule exposes its bases in sets of three, the codons. Each codon has a complementary codon called an anticodon on the tRNA molecule. When the codon of the mRNA molecule complements the anticodon on the tRNA molecule, the tRNA places the specific amino acid in that position. Then the next codon of the mRNA is exposed, and the tRNA brings the next amino acid (anticodon) and the ribosome links the two amino acids with a peptide bond. One by one, amino acids are added to the growing chain until the ribosome has “read” the entire mRNA and tRNA has brought all of the amino acids. After the protein has been synthesized completely, it is removed form the ribosome for further processing and to perform its function. For example, the protein may be stored in the golgi body before being released by the cell, or it may be stored in the lysosome as a digestive enzyme. After synthesis, the mRNA molecule breaks up and the nucleotides return to the nucleus. The tRNA molecules return to the cytoplasm to unite with fresh molecules of amino acids, and the ribosome awaits the arrival of a new mRNA molecule. The entire process Enzymes An enzyme is a protein that catalyzes, or speeds up, a chemical reaction. Enzymes are essential to sustain life because most chemical reactions in biological cells would occur too slowly, or would lead to different products without enzymes. Like all catalysts, enzymes work by providing an alternate pathway of lower activation energy of a reaction, thus allowing the reaction to proceed much faster. Enzymes may speed up reactions by a factor of many millions. An enzyme, like any catalyst, remains unaltered by the completed reaction and can therefore continue to function. Enzyme activity can be affected by other molecules. Inhibitors are naturally occurring or synthetic molecules that decrease or abolish enzyme activity; activators are molecules that increase activity. Some irreversible inhibitors bind enzymes very tightly, effectively inactivating them. Many drugs and poisons act by inhibiting enzymes. Aspirin inhibits the COX-1 and COX-2 enzymes that produce the inflammation messenger prostaglandin, thus suppressing pain and inflammation. The poison cyanide inhibits cytochrome c oxidase, which effectively blocks cellular respiration. 3D Structure In enzymes, as with other proteins, function is determined by structure. As with any protein, each monomer is actually produced as a long, linear chain of amino acids, which folds in a particular fashion to produce a three-dimensional product. Many enzymes can be unfolded or inactivated by heating, which destroys the three-dimensional structure of the protein. Most enzymes are larger than the substrates they act on and only a very small portion of the enzyme, around 10 amino acids, come into direct contact with the substrate(s). This region, where binding of the substrate(s) and then the reaction occurs, is known as the active site of the enzyme. Some enzymes contain sites that bind cofactors, which are needed for catalysis. Certain enzymes have binding sites for small molecules, which are often direct or indirect products or substrates of the reaction catalyzed. This binding can serve to increase or decrease the enzyme's activity (depending on the molecule and enzyme), providing a means for feedback regulation. Specificity Enzymes are usually specific as to the reactions they catalyze and the substrates that are involved in these reactions. Shape, charge complementarity, and hydrophillic/hydrophobic character of enzyme and substrate are responsible for this specificity. "Lock and key" model Enzymes are very specific and it was suggested by Emil Fischer in the 1890s that this was because the enzyme had a particular shape into which the substrate(s) fit exactly. This is often referred to as "the lock and key" model. An enzyme combines with its substrate(s) to form a short-lived enzyme-substrate complex. Induced fit model In 1958 Daniel Koshland suggested a modification to the "lock and key" model. Enzymes are rather flexible structures. The active site of an enzyme can be modified as the substrate interacts with the enzyme. The amino acids sidechains which make up the active site are molded into a precise shape which enables the enzyme to perform its catalytic function. In some cases the substrate molecule changes shape slightly as it enters the active site. Factors Affecting Enzyme Activity Knowledge of basic enzyme kinetic theory is important in enzyme analysis in order both to understand the basic enzymatic mechanism and to select a method for enzyme analysis. The conditions selected to measure the activity of an enzyme would not be the same as those selected to measure the concentration of its substrate. Several factors affect the rate at which enzymatic reactions proceed - temperature, pH, enzyme concentration, substrate concentration, and the presence of any inhibitors or activators. Temperature Effects Like most chemical reactions, the rate of an enzyme-catalyzed reaction increases as the temperature is raised. A ten degree Centigrade rise in temperature will increase the activity of most enzymes by 50 to 100%. Variations in reaction temperature as small as 1 or 2 degrees may introduce changes of 10 to 20% in the results. In the case of enzymatic reactions, this is complicated by the fact that many enzymes are adversely affected by high temperatures. As shown in Figure 13, the reaction rate increases with temperature to a maximum level, then abruptly declines with further increase of temperature. Because most animal enzymes rapidly become denatured at temperatures above 40·C, most enzyme determinations are carried out somewhat below that temperature. Over a period of time, enzymes will be deactivated at even moderate temperatures. Storage of enzymes at 5·C or below is generally the most suitable. Some enzymes lose their activity when frozen. Effects of pH Enzymes are affected by changes in pH. The most favorable pH value - the point where the enzyme is most active - is known as the optimum pH. This is graphically illustrated in Figure 14. Extremely high or low pH values generally result in complete loss of activity for most enzymes. pH is also a factor in the stability of enzymes. As with activity, for each enzyme there is also a region of pH optimal stability. The optimum pH value will vary greatly from one enzyme to another, as Table II shows: Inhibition Enzymes reaction rates can be decreased by competitive inhibition. Table II pH for Optimum Activity Enzyme Lipase (pancreas) Lipase (stomach) Lipase (castor oil) Pepsin Trypsin Urease Invertase Maltase Amylase (pancreas) Amylase (malt) Catalase Competitive inhibition In competitive inhibition, the inhibitor binds to the substrate binding site as shown (right part b), thus preventing substrate binding. Malonate is a competitive inhibitor of the enzyme succinate dehydrogenase, which catalyzes the oxidation of succinate to fumarate. Answer the following based on your reading: 1. What specifies the type of protein produced? 2. List the different types of RNA and describe their role in protein synthesis. 3. Who is responsible for finding the structure of DNA? 4. What structure is responsible for linking together amino acids? 5. Briefly describe the entire process of protein synthesis. Include all important molecules and locations. 6. What is a codon? 7. What is DNA, RNA and a protein? Be as specific as possible. 8. Describe and explain the central dogma of molecular genetics. 9. Are transfer RNA molecules, amino acid specific? 10. What class of macromolecules do enzymes belong to? 11. What do enzymes do in the human body? 12. How does pH affect enzymes? 13. What is inhibition? 14. How does temperature affect enzyme activity? 15. What is the active site? 16. What is the lock and key model? 17. What type of monomer makes up enzymes? 18. Enzymes work by lowering ______________________. 19. What is the induced fit model? 20. What is optimum pH? pH Optimum 8.0 4.0 - 5.0 4.7 1.5 - 1.6 7.8 - 8.7 7.0 4.5 6.1 - 6.8 6.7 - 7.0 4.6 - 5.2 7.0 2. Nucleic acids and protein synthesis 1. Do you understand how mRNA codes for amino acids? Use the chart below to decode the following strand of mRNA into amino acids of a protein. A 2. 3. 4. Date: ________________________ U G A A U U U U G A A G C U G A U The triplets that code for the amino acids on the mRNA are codons. The complementary triplet(s) on tRNA are known as anticodons. Fill in the proper triplets in the table. Amino acid codon Proline Threonine Tryptophan Leucine Arginine Histidine Glycine Serine Now use any of the codons to make up your own sequence for five or more amino acids. Don’t forget to start and stop your message. A A A C A ATA 2 TAG 3 CTT 4 TTG 5 ACG 6 GGG 7 AAC 8 CCC 9 Amino Acid U A A mRNA Codon Alanine GCU Arginine CGU Asparagine AAU Aspartic acid GAU Cysteine UGU Glutamic acid GAA Glutamine CAA Glycine GGU Histidine CAU Isoleucine AUU Leucine UUA Lysine AAA Methionine AUG Phenylalanine UUU Proline CCC Serine UCU Threonine ACU Tryptophan UGG Tyrosine UAU Valine GUU Protein synthesis Stop codon UAA Protein synthesis begins with DNA in the nucleus. Below is a DNA sequence that could code for part of a molecule of oxytocin. Write the sequence of messenger RNA (mRNA) codons that would result from the transcription of this portion of DNA. The arrow marks the starting point. ACA 1 ATT 10 After transcription, mRNA attaches to a ribosome (rRNA), where translation takes place. Each codon of mRNA bonds with an anticodon of a transfer RNA (tRNA) and each tRNA molecule bonds with a specific amino acid. The table below shows the mRNA codons and the amino acid for which they code. For example, if you were given the codon AGA, you can see from the table that these bases code for the amino acid arginine. 5. A Use you mRNA sequence form #4 to write the sequence of amino acids in this part of the oxytocin molecule. 6. How many amino acids make up this portion of the oxytocin molecule? 7. What is the purpose of the UAA codon? 8. Below is a section of a DNA molecule. Transcribe the DNA and translate it into its amino acids. ATGAAGATACGCTTTGATCGAAAAGCTACAAGAATATAA 9. Is this a complete protein, the middle, start or end? 10. How many amino acids are there? 3. Protein synthesis questions: Date: ________________________ 1. What type of monomer is linked together to assemble proteins? 2. How are proteins synthesized within a cell? 3. During translation, what is the mRNA codon paired with? 4. Myosin is a fiber found in muscle that helps muscles function. Keratin is a protein found in the skin to help give the skin its flexibility. List all the similarities and differences between those two proteins 5. In one sentence describe the role of ribosomes in protein synthesis. 6. If you were to compare the genes and proteins of a heart cell and a hair cell of the same person, what would you find? 7. What two processes involving DNA occur in the nucleus? Briefly describe what happens in each and explain why they occur in the nucleus. 8. Is the information in DNA used directly? Explain. 9. Why do we need mRNA if DNA holds the genetic information and therefore the instructions to make all the proteins the cell needs? 10. What is a synonym for Amino acid, and mRNA? 11. Which enzyme opens up the DNA double helix (unzip and unwind) during transcription? 12. Compare and contrast transcription and translation. Use a Venn diagram to assist you. 13. What process is occurring in this diagram? Explain. Where in the cell is this process occurring? 14. What is the name of the molecule being produced in the picture used in question 13? Where is the molecule going? Key 15. Look at the picture above. What process is taking place? What organelle is it taking place at? Where in the cell is it taking place? 16. Using the same picture from #15 look at the key and describe what each piece represents. 17. Look at the diagram to the left. What are the names of the amino acids in the protein chain? How many codons will there be when finished? Give the name for the molecule at A, B, C and D. Look at the area circled in black, what bases would fit there? C. A. B. D. 4. Enzymes Date: _____________________ Enzymes belong to which class of macromolecules? 1. How do enzymes work? 2. Are enzymes used up in a chemical reaction? 3. What is a substrate? 4. What is induced fit? 5. Are enzymes substrate specific? 6. Explain how enzymes lower activation energy. 7. Name two conditions that can change how an enzyme works. 8. What does it mean to be an acid a base or neutral? 9. What is a metabolic pathway and how does it relate to enzymes? 10. Look at the picture on this page. What is it and explain how it works? 11. How does hunger and eating relate to feedback inhibition? 12. Compare and contrast competitive and non-competitive inhibition. Make a drawing to show the difference. 5. Mutation questions: Date: ______________________________ 1. Name the different types of mutations and describe how they affect protein synthesis. 2. Give the amino acids from this piece of DNA: GTTGCCTATGGCAAAGCGTTT 3. Using the piece of DNA in #2 as a reference look at this mutated piece: ATTGCCTATGGCAAAGCGTTT. Find the mutation, take the mutated piece of DNA through protein synthesis and describe the type of mutation. 4. Using the piece of DNA in #2 as a reference look at this mutated piece: GTTGCCTATGGCAAAGCCTTT. Find the mutation, take the mutated piece of DNA through protein synthesis and describe the type of mutation. 5. Using the piece of DNA in #2 as a reference look at this mutated piece: TTGCCTATGGCAAAGCGTTT. Find the mutation, take the mutated piece of DNA through protein synthesis and describe the type of mutation. 6. Using the piece of DNA in #2 as a reference look at this mutated piece: ATTGCCTATGGCGAAGCGTTT. Find the mutation, take the mutated piece of DNA through protein synthesis and describe the type of mutation.