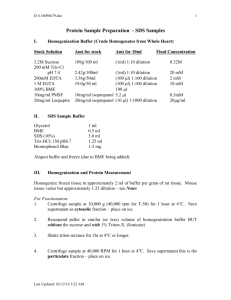
mol extraction
advertisement

Class ID: _____________ Thur or Fri. Lab: _______ Dilutions and Calculations * show enough work so I know you didn’t just copy somebody’s answers. 1. You are working with a single cell suspension of thymus cells. You have determined there are 9.0 x108 cells in your 20mL suspension. For your experiment you need 20 tubes each containing 6x106 cells/mL with a total of 5ml/tube. (Dilute with PBS). Do you have enough cells? Yes Yes. You need 100ml of 6x106 cells/mL, which is a total of 6.0 x108 cells. You have 1.5x108 cells How would you get your 20 tubes each containing 6x106 cells/mL with a total of 5ml/tube? C1 x V1 = C2 x V2 9.0x108 cells/mL x V1 = 6x106 cells/mL x 100mL V1 = 0.667mL Take approx 0.7mL from the concentrated cell solution and dilute to a total of 100 ml with PBS. Then pipet 5ml into each of your 20 tubes. 2. For gel electrophoresis you need 5 liters of a 10X TBE buffer solution, but all you can find in the lab is a 50X TBE solution. How would you make you 10x TBE solution? C1 x V1 = C2 x V2 50X x V1 = 10X x 5000ml V1 = 1000ml Add 1000ml of 50TX TBE buffer to 4000ml of dH20 3. In order to extract DNA from a hair follicle you need to add extraction buffer. Recipe for extraction buffer 25mM Kcl (MW=75 g/mol) 5mM Tris (MW=121g/mol) 1.5mM MgCl 2 (MW=15 g/mol) 0.30% NP40 0.15% Tween 20 Dilute with dH20 You need 2000mL of extraction buffer. How would you make it? M= moles/L moles/L x L = moles x grams/mole = grams Example : Needed 25mM Kcl .025moles/L x 2L = 0.05 moles 0.05 moles x 75g/mol = 3.75 grams of Kcl needed NP40 Percent x total ml = ml of Np40 needed Tween 20 Percent x total ml = ml of Tween 20 needed Add all components together and dilute to a total volume of 2000ml 4. You need to make a 1:150 dilution of a fluorescent stain for flow cytometry. What does that mean? 1 part stain to 150 parts dH20 or solution of total volume 5. You are provided with an antibody solution (Ab) that has a concentration of 800 microgram (µg)/microliter (µl). For lab it is necessary to make the following dilutions: a. 10 µl of Ab at 800 µg/µl + 190 µl of buffer to make a 1:20 dilution at __40__µg/µl. b. 20 µl of 1:20 Ab + 40 µl of buffer to make a 1:60 dilution at _0.667_____µg/µl. c. 5 µl of 1:60 Ab + 5 µl of buffer to make a _1:2____ dilution at _0.334___µg/µl. d. 10 µl of 1:60 Ab + 90 µl of buffer to make a ___1:10__dilution at _0.0667___µg/µl. e. 10 µl of 1:60 Ab + 40 µl of buffer to make a __1:5____dilution at _0.1334___µg/µl. f. 10 µl of 1:60 Ab + 10 µl of buffer to make a ___1:2___dilution at ___0.334__µg/µl o All dilution factors are from the original 800 µg/µl concentration. o You can use C1 x V1 = C2 x V2 to get the concentrations or you can use the dilution factor. o To determine dilution factor: Take the initial concentration and divide it by the final concentration Ex: Dilution of a 600 µg/µl solution to 5 µg/µl is a 1:120 dilution. 600/5 is 120. o To use the dilution factor to determine concentration: Divide the initial concentration by the second number in the dilution factor and then multiply by the top number to get the new concentration 1:60 dilution of a 600 µg/µl Ex: 600 µg/µl divided by 60 = 10 x 1 = 10 µg/µl 6. 3 oz. of vodka was added to 6 oz. of orange juice. What dilution was made of each component? If the vodka were 100 proof (50% ethanol), what would be the final concentration of alcohol in the drink? 3 oz of vodka into a total volume of 8oz. That is a 1:2.6 dilution of vodka. 50% of 3oz = 1.5 oz of Ethanol 1.5oz is what % of 8oz = 18.75% 7. Convert the following. 10 gram = ___10000__ mg 119mL = _0.119_ L 0.9M = __900_____ mM 52g = _520000__ g 4.5mL = ___4500____ L 0.21 meters = _210____ mm 105 gram = ____0.105___ Kg 150µg = __1.5 x 10-7__kg 8.You weighed out 15.5 g of CaCl2 and diluted it to 350 mL with distilled water. What is the percent solution (W/V)? percent solution = g/ml x 100 (15.5g / 350ml) x 100 = 4.4%