Chapter 4: DISSOLVED OXYGEN MEASUREMENT
advertisement

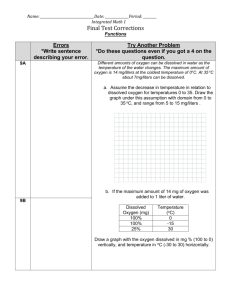
Measuring Dissolved Oxygen using the Winkler Method DISSOLVED OXYGEN (DO) INTRODUCTION DO determination measures the amount of dissolved (or free) oxygen present in water or wastewater. Aerobic bacteria and aquatic life such as fish must have DO to survive. Dissolved oxygen levels can be negatively affected by pollutants. Microorganisms may flourish when organic pollutants from wastewater or runoff enter an aquatic environment. Feeding off of these nutrients, the microbes thrive thereby increasing the demand of DO which ultimately leads DO decreases. Dissolved Oxygen is measured in parts per million (ppm). 5-6 ppm Sufficient to most aquatic species 2-4 ppm Stressful to most aquatic species < 2 ppm Fatal to most species What is the Winkler Method? The Winkler Method is a technique used to measure dissolved oxygen in freshwater systems. Dissolved oxygen is used as an indicator of the health of a water body, where higher dissolved oxygen concentrations are correlated with high productivity and little pollution. This test is performed on-site, as delays between sample collections and testing may result in an alteration in oxygen content. How does the Winkler Method Work? The Winkler Method uses titration to determine dissolved oxygen in the water sample. A sample bottle is filled completely with water (no air is left to skew the results). The dissolved oxygen in the sample is then "fixed" by adding a series of reagents that form an acid compound that is then titrated with a neutralizing compound that results in a color change. The point of color change is called the "endpoint," which coincides with the dissolved oxygen concentration in the sample. Dissolved oxygen analysis is best done in the field, as the sample will be less altered by atmospheric equilibration. Equipment and Material: Gloves Safety Goggles 60 mL glass water sampling bottle Magnesium Sulfate Solution Alkaline Potassium Iodide Azide Sulfuric Acid Sodium Thiosulfate Starch Indicator Solution Titrator Direct Reading Titrator Thermometer Procedure: 1. Rinse sampling bottle with the water being sampled 2. Tightly cap the bottle and submerged to desired depth 3. Remove the cap from the bottle allowing bottle to fill. Tap the sides to dislodge possible air bubbles 4. Replace the cap 5. Remove cap and immediately add: a. 8 drops of Maganous Sulfate Solution b. 8 drops of Alkaline Potassium Iodide Azide 6. Cap the bottle and mix by inverting several times. A precipitate will form. 7. Allow the precipitate to settle (below shoulder of the bottle) 8. Add: 8 drops of Sulfuric Acid 9. Cap and gently invert the bottle to mix the contents until the precipitate and reagent have total dissolved. The solution will be clear yellow to orange if the sample contains DO. NOTE: the sample is now Fixed and contact between the air and sample will not affect the results. This allows you to hold samples for titration at a later point and duplicate the analysis of a sample. 10. Fill the titration tube the 20 mL line 11. Depress plunger of the Titrator 12. Insert Titrator into the pig in the top of the Sodium Thiosulfate titrating solution 13. Invert the bottle and slowly withdraw the plunger until the large ring on the plunger is opposite the zero(0) line on the scale 14. Turn the bottle upright and remove the Titrator 15. Insert the tip of the Titrator into the opening of the titration cap 16. Slowly depress the plunger to disperse the titrating solution until the yellowbrown color changes to a very pale yellow. Gently swirl the tube during the titration to mix the contents. 17. Carefully remove the Titrator and cap. Do not disturb the Titrator plunger. 18. Add: 8 drops of Starch Indicator Solution. The sample should turn blue. 19. Cap the titration tube. Reinsert the tip of the Titrator into the opening of the titration tube cap. 20. Continue titrating until the blue color disappears and the solution becomes colorless. 21. Read the test results directly from the scale where the large ring on the Titrator meets the Titration Barrel. Record as ppm Dissolved Oxygen. Each minor divisions in the Titrator scale = 0.2 ppm 0.1 mL Sodium Thiosulfate used = 1 ppm Dissolved Oxygen (or 1 mg DO per L of water) Example: 1.2 mL Sodium Thiosulfate used = 12 ppm Dissolved Oxygen Water temperature affect the amount of dissolved oxygen an aquatic ecosystem can hold. To determine the percent saturation, locate the temperature (deg C) of the water sample on the top scale. Locate the corrected dissolved oxygen concentration on (ppm) on the bottom scale. Draw a straight line between the two points. Read the % saturation where the line crosses the % saturation scale.