Ammonia vs. Ammonium
advertisement

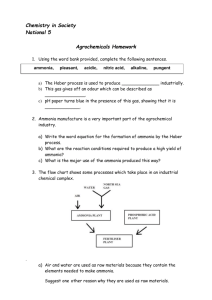
Pre-AP Chemistry Instructor: Mrs. Blakely Name ___________________________________ Period _________ Due Date _____________ Laboratory Activity: Ammonia and Ammonium Ammonia is a common gas (NH3) that has a sharp odor. Salts of ammonia have been known since the 13th century. Ammonia was produced by the action of alkalis on sal-ammoniac. Gaseous ammonia was isolated in 1774 by Priestly, Scheele identified the nitrogen content in 1777, and Berthollet identified its composition in 1785. Ammonia is a gas made from nitrogen and hydrogen in the Haber Process that was patented in 1910. Most people are familiar with it as a household cleaner, which is generally a 5-10% aqueous solution by weight. Household ammonia is also considered to be a weak ammonium hydroxide solution. Ammonium salts can be found as a variety of industrial and consumer products. Solutions of these salts react with strong bases to produce ammonia, as example, in the following reaction: NH4Cl (aq) + NaOH (aq) NH3 (g) + H2O (l) + NaCl (aq) Some ammonium salts undergo a natural decomposition in air to release ammonia gas. Ammonia will react with moist neutral litmus paper, turning it blue, as an indication of the base formed. This activity will allow you to study ammonia as a compound and the ammonium ion. Materials- household ammonia, NH4Cl (s), NaOH (aq), test tubes with rack, test tube holder, stoppers, burner, sparker, neutral litmus paper, 10 ml graduated cylinder, NH4NO3 (s), (NH4)2CO3 (s), fertilizer, window cleaner. Methods1. Fill a test tube 1/3 full with ammonia. Observe the odor and test with a moist litmus paper holding it above the surface of the liquid in the test tube. 2. Gently heat the test tube in the burner, observe the odor again and test with a moist litmus paper. Discard this solution in the sink. 3. Observe the odor of the ammonium chloride(s) sample provided. Test the solid by holding a moist litmus paper above it. 4. Dissolve a small amount (about a gram) of ammonium chloride(s) in a test tube 1/3 full of water (swirl or stopper). 5. Observe the odor, test with moist litmus. Add 3 ml of NaOH (aq), mix, gently heat, then observe the odor, test with moist litmus again. Discard the products of the reaction in the sink. 6. Repeat steps 3, 4, and 5 individually using the (NH4)2CO3, NH4NO3, and the fertilizer in place of the ammonium chloride. 7. Observe the sample of window cleaner. Test in anyway feasible to determine ammonia content. Note what tests you used and the results. Observations (60 pts) Note: No more than two words for odor or litmus. Litmus is only red or blue. (ex. for odor- salty, musty, very pungent, mildly acidic; for litmus: red, dark blue, instant blue) 1. NH3 (aq) sample- odor- ________________________ litmus- _____________ 2. NH3 (aq) sample with heat- odor- ________________________ litmus- _____________ 3. NH4Cl (s)over---- odor- ________________________ litmus- _____________ 4. NH4Cl (aq)- odor- ________________________ litmus- _____________ 5. NH4Cl reaction- odor- ________________________ litmus- _____________ 6. (NH4)2CO3 (s)- odor- ________________________ litmus- _____________ 7. (NH4)2CO3 (aq)- odor- ________________________ litmus- _____________ 8. (NH4)2CO3 reaction- odor- ________________________ litmus- _____________ 9. NH4NO3 (s)- odor- ________________________ litmus- _____________ 10. NH4NO3 (aq)- odor- ________________________ litmus- _____________ 11. NH4NO3 reaction- odor- ________________________ litmus- _____________ 12. fertilizer (s)- odor- ________________________ litmus- _____________ 13. fertilizer (aq)- odor- ________________________ litmus- _____________ 14. fertilizer reaction- odor- ________________________ litmus- _____________ 15. window cleaner- _________________________________________________________ ___________________________________________________________________________ Questions (40 pts) 1. Name two ways that ammonia is different from ammonium? 2. Why is fertilizer used as a sample in this lab? 3. Ammonia plays a very minor role in window cleaners. Explain its use. 4. Why is the heating of the test tube solutions done in this lab? 5. What are two basic safety precautions to be taken when using ammonia in the home or lab?