Teacher Preparation Notes for Metal Identification Lab
advertisement

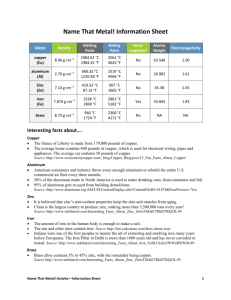
Teacher Preparation Notes for Metal Identification Lab Background: Many methods are used to identify a piece of metal. Some common methods used for field identification are surface appearance, chemical tests, and the use of a magnet. Sometimes it is possible to identify metals by their surface appearance. Copper and copper alloys will have an orange-brown or yellowish appearance, while tin, aluminum, iron, and zinc will have a dull or shiny gray-silver surface. When the surface appearance of a metal does not give enough information to allow positive identification, other identification tests must be done to identify the metal. All metals can be placed into two groups --- ferrous or nonferrous. Ferrous metals are magnetic and include iron. Other metals are nonferrous. Reaction with strong acids such as hydrochloric acid can be used for metal identification. Zinc reacts immediately and violent with HCl. Tin shows little or no reaction with HCl, and aluminum has a delayed reaction with HCl. Data Table: Metal Aluminum Copper Appearance Gray, dull, sometimes shiny Yellow to orange-brown Magnetic HCl No Delayed reaction No No reaction Iron Dark Yes Delayed reaction Tin Dull, yellowish metal No Little, if nay reaction Zinc Shiny, Dense (heavy) metal No Immediate violent reaction Time: Teacher preparation – 20 minutes 1 Class time – 45 minutes Materials: Samples of metals (iron, aluminum, tin, zinc, and copper) 2- 250 ml beakers (per lab group) Disposable dropper (one per group) HCl Student Safety Issues: Review students on proper dress for lab --- aprons, goggles, and gloves. Also review students on location of sinks and eye washes for flushing off chemicals and the care and use of acids. Disposal of Materials: Hydrochloric acid may be poured down the sink and flushed with excess water. Hint: Set up all lab supplies the day before lab on a lab cart or an area of the lab accessible to all students. Answer to Questions: 2 COLOR YELLOW to ORANGE-BROWN NOT Yellowish MAGNETIC YES COPPER or COPPER ALLOY IRON or IRON ALLOY NO REACT WITH ACID FAST? NO NO TIN 1. 2. a. copper ZINC ALUMINUM b. iron References: Metals color test – http://www.muggyweld.com/colortest.html Metal Identification http://www.tpub.com/content/construction/14250/css/14250_21.htm Metal Properties – http://www.angelfire.com/my/welding/metal.html 3