extraction of caffeine

EXTRACTION OF CAFFEINE
Denise Traniello, O’ Bryant School of Math and Science docace@comcast.net or dtraniello@boston.k12.ma.us
Sotiris Pentidis Boston Community Leadership Academy, spentidis@hotmail.com
OVERVIEW
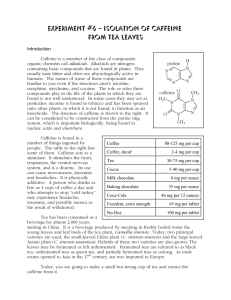
Isolating them from plants can produce a number of chemicals and/or drugs. This lab will isolate caffeine from tea.
INTRODUCTION
The main topics covered:
1.
States of matter
2.
Mixtures and separation of mixtures
3.
Intermolecular interactions
4.
Polar and non-polar compounds
5.
Energy and Change
6.
Heat and Temperature
7.
Covalent compounds
8.
Isolation of natural products
The main advantage of this approach is that it combines both the experimental extraction of the caffeine, with a series of simulations.
The experiment itself shows a very practical application of the above principles and help students to become familiar with a number of different experimental techniques. It can also be converted to a project studying and comparing different types/brands of teas and coffee.
On the other hand the simulation will help students understand what happens in microscopic level and be able to modify the different parameters and see their effect and the role they play in the process.
INTENDED AUDIENCE
The unit will be part of a project for honors students and/ or students that take organic chemistry. It will basically serve as a review of topics such as, mixtures, separations methods, temperature and as a way to introduce and comprehend the concept of polar and non polar and intermolecular interactions. It can also be a part of a larger unit of food or applied chemistry.
It addresses the following Massachusetts state frameworks which are the basis for the MCAS as well as the Boston Public School science standards.
Matter 1.1 and 1.2
Chemical Bonding 4.5
Kinetic Theory 6.2
Heat and Temperature 10.3
ADJUSTMENT / ADAPTATION
There are two major adaptations that can be done so that the unit can be taught for different level of students.
First advanced students who had enough laboratory experience can use this unit to study different teas and coffee samples. For introductory chemistry students can only do one sample of tea.
Secondly students of introductory chemistry will use the simulation more as a demonstration tool and won’t try to change any of the parameters.
PLACEMENT TO CURRICULUM
Unit adheres to the Massachusetts Frameworks.
This unit follows the discussion about polarity and intermolecular interactions.
Students have been exposed to the concepts and most experimental techniques needed.
This unit is comprised by both a lab assignment and a lecture
/computer demonstration part. We believe that both the wet lab and the simulab will enhance the understanding of the subject from two different angles, the microscopic and the macroscopic.
Also it is part of the drive to introduce students to the use of chemistry in everyday life.
TIME
1 period prelab: Give the lab to the students and outline the procedure and materials and equipment, prelab questions
2-3 periods for the lab.
1 period post lab: go over the results, calculations.
1 period: Talk about polar and non-polar interactions, explain how the program works
1 period performing the simulation
1 period discussing the outcome of the simulation
(Time can be adjusted depending upon length of class period/double lab period. We elected to use a single 45 minute period.)
RESOURCES
Materials: rotovapor machine or hot plate
1000-mL beaker ice (cubes or crushed) petri dish glass stirring rod
500-mL separatory funnel expendable rubber tubing regular funnel goggles filter paper three 400-mL beakers basin for ice
Tea with caffeine
100-mL graduated cylinder
NaHCO
3 ring stand with ring dichloromethane/propanol hood
250-mL Erlenmeyer flask
Na
2
SO
4
(anhydrous) aspirator or vacuum setup rubber tubing round-bottom flasks gloves
Software and Hardware requirements
1 computer for 2-3 students
VMDL program
Bibliography and web sites
Microscale Chemistry for High School, Z. Szafran, R.
Pike, M. Singh Vol 2
Holt Chemistry p 194-195 and page 386
Murray, Scott D.; Hansen, Peter J. The Extraction of
Caffeine from Tea: An Old Undergraduate Experiment
Revisited J. Chem. Educ. 1995 72 851.
http://departments.oxy.edu/tops/Caffeine/caffprepost.
htm
ELECTRONIC EQUIPMENT
We need a computer lab with enough computer so that each group of 2-3 students have one computer.
GOALS and OBJECTIVES
Students will be able to perform an extraction , filtration and a sublimation of caffeine
(This can be achieved by performing the wet lab)
Students will be able to describe the effect of the interaction between particles to mixing and phase creation
(This can be achieved by performing VMDL simulation)
Instructional Activities
1 st Period: Hook Demo: Electric conductivity between two phases
(HCl
(g)
in hexane plus water and check the conductivity)
Discuss the demo.
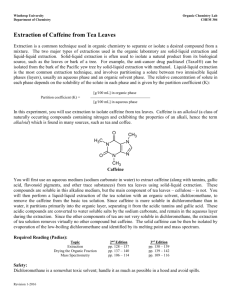
Give them the Lab and discuss about the procedure and about caffeine (what substance is it, where can it be found, its formula etc ).
Review also some vocabulary terms like , mixture, polar non polar, phase, sublimation, filtration. If there is time left they can start the lab prep
2 nd and 3 rd period: performing the lab
4 th period: Discussing the results and the calculations and going over the postlab questions. Students have to write a lab report.
5 th period : Demo Using the water program to show what happens when a polar and a non polar substance is dissolved in water.
Teacher will introduce the VRML program and especially the
Universal part of it, explaining how one can change the interactions between particles
6 th period: Students will run a simulation, simulating the extraction of caffeine from polar liquid.
The simulation consists of two phases of liquids (red and green particles) with the caffeine molecules (blue) dissolved in one of them
(red).
Students will start the simulation by switching of the attraction between the blue and green particles , by editing the simulation file.
As always before they start the simulation they will be asked to predict what will happen.
Then they will introduce different attraction strength in the interaction between blue and green particles and they will study the effect will have on the rate of extraction. Students also will vary the temperature for given attraction strength to study the effect it has in the rate of extraction.
7 th period: Students will discuss the results of their simulations.
LABORATORY RUBRIC
4 3 2 1
Definition Demonstrate the required knowledge and skills.
Comprehension Curriculum expectations are fully or consistently met.
Applications Extends skills to innovative solutions.
Demonstrate most of the required knowledge and skills.
Most of the curriculum expectations are met. Few errors.
Transfers skills to familiar and new situations.
Communication Explanations are complete and appropriate with
Problem
Solving evidence of a variety of contexts.
Learning strategies are modified and new ones created.
Communication is clear and precise.
Appropriate learning strategies are used.
Limited learning strategies are used.
From a teacher’s perspective, are students able to work independently, with limited assistance, or assistance?
Demonstrate some of the required knowledge and skills.
Half the specified curriculum expectations are met.
Transfers some skills between familiar situations.
Explanation is appropriate but incomplete.
Has not demonstrated the required knowledge and skills.
Only a few of the curriculum expectations are met.
Does not yet transfer skills.
Explanation is incomplete or inappropriate.
Very limited learning strategies are used.
Extraction of Caffeine
Homework
1.
What is the formula of caffeine?
2.
Why is caffeine considered a drug?
3.
Can you give examples of extraction in everyday life?
4.
Judging from your experiment which interaction is stronger, the caffeine with the polar phase or the caffeine with the non polar phase explain?
5.
How use of soap compare with extraction, what do they have in common and what is different.
6.
EXTRACTION OF CAFFEINE SIMULATION
Objective:
You will be able to describe how attraction and repulsion affect the distribution of a liquid in two phases.
You will be able to determine whether temperature affects the rate of extraction
Procedure:
1.
Open My Files choose the Extraction file and
Edit it .
Switch off the attraction between the blue and the green particles. So now all the particles repel each other
Q.1 What do you expect to happen, explain
2.
Run the simulation for 10000 time units and record what happens .
3.
Edit again the Extraction file but this time introduce a –2 attraction between blue and green particles
Q.2 What do you expect to happen now after running the simulation, explain
4.
Run the simulation for 10000 time units and record what happens .
5.
Edit again the Extraction file but this time introduce a –5 attraction between blue and green particles
Q.3 Is the attraction stronger or weaker now? Do you expect the blue particles to migrate faster now into the green phase or not why?
6.
Run the simulation for 10000 time units and record what happens.
7.
Keeping the same attraction factor as before, run the simulation with three different temperatures:
0.1, 0.5, 1 for 5000 time units. Record your observations.
Q.4 What is the effect of increasing the temperature on the extraction rate?
Write a write-up summarizing the simulation , your observations , answering the questions and explain why your predictions agree or disagree with the results
Extraction of Caffeine from Tea
Prelab Questions
1.
What is the correct method for separating immiscible liquids?
2.
When the two layers are put in the separatory funnel what property will explain which phase is on the top and which one on the bottom?
3.
What is the use of Na
2
SO
4
?
4. (a)What is sublimation?
(b)How does sublimation differ from vaporization?
(c)How does sublimation differ from evaporation?
5.
Why it is necessary to “thoroughly” dry the beaker after the sublimation process is finished?
Lab: Extraction of Caffeine from tea
Question: What is the percentage of caffeine in tea
Materials: rotovapor machine or hot plate
1000-mL beaker ice (cubes or crushed) three 400-mL beakers basin for ice
Earl Grey tea petri dish glass stirring rod
500-mL separatory funnel expendable rubber tubing regular funnel goggles filter paper rubber tubing round-bottom flasks
100-mL graduated cylinder
NaHCO
3 ring stand with ring dichloromethane/propanol hood
250-mL Erlenmeyer flask
Na
2
SO
4
(anhydrous) aspirator or vacuum setup gloves
organic waste can kitchen mitts
PROCEDURE
1. Put on goggles.
2. Set up the separatory funnel in advance. Place the ring stand in the hood. It is desirable to place rubber bumpers on the iron ring so as to protect the glass from breakage. The rubber for this will come from expendable rubber tubing. Cut a short section of tubing, then cut it open lengthwise, so that it can be wrapped around the ring.
Place these bumpers approximately 120 o
from each other on the iron ring, and this will protect the funnel against damage when it is placed in the iron ring.
3. Prepare a basin of ice for cooling the beaker of tea later.
4. In a hood start heating the water in the rotovapor machine (or in a beaker on the hot plate, if there is no rotovapor machine).
5. The objective is to produce the greatest number of grams of caffeine per tea bag.
Students are given this objective in advance, so they can do research in the library, on the
Internet, and by talking to experts, to identify promising teas for extraction of the most caffeine. Start with four tea bags.
6. Four tea bags are to be massed. It is important that tea leaves not leave the bags, so the bags should not be punctured, but strings and tags should be cut off and paper wrappers removed before massing.
7. Using the graduated cylinder, measure 100mL of distilled water into a 400-mL beaker.
8. Mass 2.0 g of NaHCO
3 and use glass rod to stir into beaker of water.
9. Submerge tea bags in the solution in the beaker.
10. Place a clean, dry petri dish on the top of the beaker to prevent splattering. Thus covered, the beaker should be placed on the turntable of the microwave oven. Set microwave oven for 100% power for a duration of 1 minute. Then start the oven. After one minute, if boiling is observed, then go to next step. If no boiling is observed, irradiate for another 1-minute interval, and continue to do so in 1-minute intervals until boiling is observed.
11. Decant the liquid to a 400-mL beaker and examine the liquid to see if any tea leaves have escaped the bags. If the liquid is free of tea leaves, then proceed to the next step.
Otherwise, repeat steps 6 through 11.
12. Cool the tea by setting the beaker in a basin of ice.
13. Ensure separatory funnel valve shut. Using an ordinary funnel, pour the tea into the separatory funnel.
14. Put on gloves.
15. The separatory funnel should be placed in the hood if it has not been already.
16. Using a graduated cylinder, measure 25 mL of dichloromethane or propanol.
Dichloromethane and the tea mixture can form an emulsion difficult to separate if allowed to be agitated, so slowly pour the dichloromethane into the separatory funnel.
17. Avoid shaking the separatory funnel too vigorously. Remove the top of the separatory funnel (if it is not already removed), and gently tilt the mixture back and forth, or else a bothersome emulsion will form.
18. After the bottom layer is removed from the separatory funnel, it must pass through a drying agent (such as Na
2
SO
4
). The purpose of this second filtration setup is to remove any H
2
O from the bottom layer. Set up the Erlenmeyer flask close by the separatory funnel, and place a conical funnel in the Erlenmeyer flask. Line the conical funnel with filter paper. Place a small amount of dichloromethane on the filter paper so that it will adhere to the sides of the funnel. This setup will be used in the next step to remove
Na
2
SO
4
.
19. Place an Erlenmeyer flask under the separatory funnel to catch the bottom layer.
Gently open the valve to allow only the bottom layer to pass through. Turn the valve to the shut position before any of the top layer passes through. Add anhydrous Na
2
SO
4
to the liquid and stir. Pour the contents of this flask through the paper-lined conical funnel so as to remove the Na
2
SO
4
.
20. Set up the rotovapor machine. (Omit this step if you will be using a hot plate instead.) Ensure tubing is hooked up for aspirator (or vacuum) and for cooling water, including a secure hose leading to a drain. Turn on the cooling water.
Transfer the filtrate to a round-bottom flask, and attach this to the appropriate end of the rotovapor machine. Ensure a receiving flask is also in place. Apply a partial vacuum by opening the vacuum valve, or, if you lack a vacuum valve, set up an aspirator to provide a partial vacuum. Shut the valve at the top of the condenser. Start the rotation of the sample flask. Gently lower the sample flask into the water, but if the solvent boils too violently, elevate the sample flask and lower the temperature of the water, then try again.
21. If you lack a rotovapor machine, do the following: set up a heating bath in a 1000-
mL beaker on a hot plate in the hood. Place the filtrate in an Erlenmeyer flask, and using kitchen mitts, lower the Erlenmeyer flask until its bottom is in contact with the hot water.
This will provide heat to evaporate the solvent. Any time the filtrate begins to boil, lift the flask, then lower it again. Eventually the solvent will be driven off and a solid will be left behind.
22. With a spatula, remove the solid left behind and mass it. This solid is principally caffeine. Record this for comparison with other teas.
23. If this is the last tea sample to be tested, then commence clean up.
DATA
Tea Brand Mass of Tea Mass of
Caffeine
%of Caffeine
ANALYSIS QUESTIONS
1.
What is the importance of intermolecular forces in the dissolving process.
2.
What is the difference between the intramolecular and intermolecular forces?
3.
Why you are using the above procedure instead of just simply evaporating the water.
4.
Which one has more caffeine, tea or coffee?
5.
What are your sources of error?
ASSESSEMENTS
Apart from the informal on going assessment (discussion with students, observation of their experimental skills) we have some formal assessments.
Homework: In which we try to assess some previous knowledge about caffeine, the use of extraction and the factors that affect the intermolecular interactions
Formal Lab Report: Students will write a formal lab report demonstrating their understanding of their experimental method and the interactions that take place.
Write up for the simulation : Students will write an assignment in which they will explain the main principles of the simulation, their predictions and the results , compare them and explain why they agree or not
EDUCATIONAL ISSUES
The Boston Public Schools offer an array of challenges for the high school science teacher.
1. Laboratory equipment and computers varies from school to school.
2. There are no “passing” benchmarks in science. Students not meeting benchmarks in ELA or math must summer school.
3. Boston is heavily minority district and with it are problems associated with any large, urban school district. a. The majority of students are black, Latino, Asian. b. English is not the native language of many students. c. English is not spoken at home. d. Many BPS students come from foreign countries. e. Income level at or slightly above poverty level (free and/or reduced priced breakfast/lunch) f. A number of students come from single parent households, are being raised by relatives, or are emancipated minors. g. A number come from households with parents having limited education – not a high school grad.
Many of our students are successful and take advantage of the opportunities available through the NIH and NSF and local colleges. Our challenge is dealing with the students are not successful. How do we take that complicated topic and make it understandable? How do we convince students that our course of student is relative to the big picture?