9. lecture
advertisement

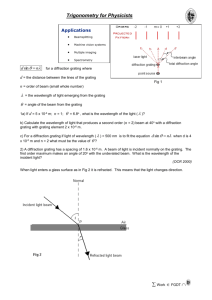
9. lecture Transient Grating Spectroscopy As the energy of the excited molecule will be partly utilized for physical/chemical purposes and partly for heating up the surrounding matrix (solution), the thermal detection methods can be universally used for studies of photoexcited systems. Among the photothermal spectroscopies, the transient grating (TG) spectroscopy is a very powerful and unique tool for measurement of relaxation times of excited states, enthalpy change and molecular volume and conformational changes with high sensitivity and excellent time resolution. The different lasers play determining role in the instrumentation and it could not have been established in lack of sophisticated lasers. Basic idea. The TG is an optical heterodyne detection technique characterized by mixing of several waves (Fig. 10.1). When two coherent laser beams with parallel polarization are crossed at a spot within the time of coherence, the interference between the waves creates a sinusoidal modulation of the light intensity in the sample. The light-matter interaction and subsequent possible photophysical and photochemical processes change the optical properties of the material in the bright region which results in spatially modulated refractive index or absorbance pattern with grating fringe spacing Λ. The locally generated refraction index grid remains constant for a period of time determined by several dissipating processes (e.g. diffusion, sound, chemical processes etc.) which decrease the contrast and the visibility of the fringes. The interference pattern is detected by a third continuouswave laser beam. This probe beam is introduced under Bragg-angle conditions (phase matching direction) to the grating. A photomultiplier collects the intensity fluctuations of the probe light (TG signal). Since the intensity of the diffracted probe light is related to the amplitude of the peak-to-null modulation, the temporal dependence and the intensity dispersion in the probe wavelength should reflect the dynamics and the optical properties of the active species. This qualitative description can be quantified by assumption of plane waves of the two pumping laser beams of electric fields E1( 2) E10( 2) (t ) exp i k1( 2) r t (9.1) where ω is the optical frequency, k1(2) is the propagation vector for wave 1 (2) and E01(2)(t) is the vector of the electric field including the polarization and the temporal profile. The optical interference pattern is described as I ( x, t ) I1 (t ) I 2 (t ) 2 0 c n E1 (t ) E2 (t ) exp( i q x) (9.2) where I1(2) = ε0·c·n· |E1(2)|2 is the light intensity in each beam (ε0, c and n are the dielectric constant of the vacuum, the speed of light in the medium and the refractive index, respectively), q is the grating wave number vector: q = k1 - k2 and x is the coordinate that transverse to the average direction of the excitation (pumping) beams (Fig. 10.2). By assuming that the directions of the polarization are parallel to each other and that the amplitudes of the two electric fields (E10 = E20) and therefore the intensities (I1 = I2 = I0) are equal the optical interference may be written as I ( x, t ) 2 I 0 f (t ) 1 cos(q x) where f(t) is the temporal profile of the laser beam. The fringe spacings, Λ is given by 1 (9.3) pump Θ 2 sin 2 (9.4) where Θ is the angle between the two exciting beams (θ ~ 20o) and λpump is the wavelength of the excitation. The light-matter interaction creates a sinusoidal modulation of the refractive index and (through the Kramers-Kronig relationship) the absorbance. The grating is monitored by the diffraction efficiency of a continuous wave (cw) probe beam or another time-delayed laser pulse (Fig. 9.2). For the thick grating, the probe is brought in at an appropriate angle to satisfy the phase matching condition (the Bragg condition). Under a weak diffraction condition, the diffraction efficiency and thus the transient grating signal (ITG) is approximately proportional to the square of the variations in the refractive index Δn and absorption Δk 2 k d 2 d n d k 2 2 I TG exp sinh 2 sin n k (9.5) cos Θ 2 cos Θ probe cos Θ where d is the thickness of the sample, α and β are constants which represent the sensitivity of the system and λprobe is the wavelength of the probe beam. The first term describes the effect of the changes of the refractive index (the phase grating) and the second term results from the absorption change (the amplitude grating). Principal modes of grating. There are various causes of changes of the refractive index (or absorption) upon light irradiation. 1) Thermal grating. After the nonradiative transition of the excited states, the temperature is raised due to the released energy. The density of the matter (solution) is altered by the heating effect which creates the changes of the refractive index (Δnth), thus the thermal grating dn N W h (9.6) nth dT cp where dn/dT is the temperature dependence of the refractive index of the solution, N is the number of the excited molecules per unit volume (mol/cm3), W is the molecular weight (g/mol), h·ν is the photon energy of the excitation light (J/mol), cp is the heat capacity at a constant pressure (J/mol/K) and ρ is the density of the solution (g/cm3). If the amount of the released heat is changed by a factor Φ due to a chemical reaction, the change of the refractive index change of the sample should be nth N W h dn . Φ dT cp (9.7) The fraction of energy released as heat may be given by Φ 1 H , h (9.8) where ΔH is the enthalpy change of the reaction and η is the quantum yield of the reaction. We can determine η ·ΔH by comparison of the TG signal intensity of a sample with that of a calorimetric reference under the same experimental conditions. The ration of the refractive index changes for the sample (Δnth) to that of the reference (Δnthref) under the same experimental conditions is given by 2 n th Φ. ref n th (9.9) When the rate of the chemical reaction is not fast enough compared to Dth·q2 (the rate of heat diffusion), the time evolution of the heat releasing process Q(t) should be taken into account and the time profile of the thermal grating signal intensity is given by n th (t ) dn W dQ(t ) exp Dth q 2 t , dT c p dt (9.10) where * denotes the convolution integral. Hence, we can measure the time dependence of the enthalpy change ΔH during a thermodynamic reaction. According to the Clausius-Mosotti equation n2 1 n2 2 V solvent , 3 0 Vsolvent N (9.11) where n is the refractive index of the solution, ε0 is the permittivity of the vacuum and α and αsolvent are the molecular polarizability of the species (reactant/product) and the solvent molecule, respectively. Any changes of the polarizability (α) or of the molar volume (V) of the reactant will cause changes of the observed refractive index. We can decompose the change of the refractive index (Δn) into changes of the polarizability (Δα) and of the molecular volume (ΔV). 2) Population grating is the change of the refractive index (Δnpop) which is induced by a difference of molecular polarizability between the reactant and product molecules. npop n N n02 2 9 0 n0 2 . (9.12) n was calculated from Eq. (9.11), n0 refers to the refractive index of the reactant solution before the excitation and Δα denotes the change of the polarizability between the reactant and product. 3) Volume grating is the change of the refractive index (Δnvol) which is induced by a difference of molecular volume between the reactant and product molecules. Here, the differential quotient nvol n N n02 2 V V 9 0 n0 2 solvent Vsolvent V . (9.13) Hence, by isolating the volume grating in the TG signal, we can measure both the volume change and the partial molar volume. The terms of population and volume grating is called species grating. The population and volume gratings are related to the corresponding change of the absorption spectrum by the Kramers-Kronig relation. The discussion is beyond our possibilities. Instrumentation. The four-wave mixing technique is shown on Figure 9.3. After photoexcitation of the sample by a grating pump light, a probe field Eprobe(t) is created by the diffraction of the probe light. If it is interfered with a local oscillator (LO) field ELO, the observed light intensity is 3 I TG (t ) Eprobe (t ) ELO 2 I LO n(t ) I pump I probe cos . (9.14) where Δn(t) is the change of the refractive index, Δφ is the phase difference between the local oscillator (LO) and probe light fields and Ipump, ILO, and Iprobe are the intensities of the excitation, LO, and probe light, respectively. It is assumes that the refractive index change is the dominant source of the TG signal, and the probe field is weak compared to that of the local oscillator: |ELO| >> |Eprobe|. Since the local oscillator light intensity is constant, ILO provides constant background. The second term on the right-hand-side of Eq. (9.14) represents the dependence of the TG signal on the relative phase difference between the LO and probe fields that can be adjusted to minimum and maximum values. Their difference offers the TG signal attributed to the sample (Fig. 9.4). Applications. 1) Temperature-dependence of the refractive index of water. Figure 9.5 shows the temperature-dependence of the refractive index obtained from TG signal of the aqueous solution of Evans blue. According to Eq. (9.10), the amplitude determines dn/dT and the observed lifetime is characteristic of the heat diffusion coefficient (Dth) and the grating wavenumber (q). The refractive index of the water depends strongly on the temperature and dn/dT vanishes around 0 oC. 2) Molecular consequences of charge separation in bacterial reaction center (Fig. 9.6). The lightinduced charge separation in bacterial reaction center protein (PQ + hν → P+Q-) induces transient grating. The thermal grating can be observed but is not substantial as not much heat is produced due to the high the yield of photoconversion. The population grating and the volume grating are the dominant modes of grating. The population grating follows the kinetics of charge recombination (P+Q- → PQ) both in QA (lifetime ~100 ms) and in QB (lifetime ~1 s) active states of the RC. If, however, the native ubiquinone is removed from the QA binding site, a much smaller TG signal is observed with ~ 40 μs lifetime that is much larger than the lifetime of the P+BPh- charge separated state ( ~ 10 ns). The corresponding population grating signal is out of the range of our time resolution and cannot be seen. The observed slower TG signal is sensitive to the volume change and characteristic of the relaxation of the charge separated state of the protein (volume grating, Table 9.1). 3) High-harmonic interferometry of dissociating Br2 molecule. By exciting a molecule with an intense femtosecond laser pulse (~1014 Wcm-2), an electron wave packet is extracted from one of the valence orbitals and driven back to interfere with the remaining bound electronic state. If the electron recombines, it emits extreme ultraviolet (XUV) radiation in a train of attosecond pulses, a process known as high-harmonic generation (HHG). All molecules in the sample radiate coherently in a phase-matched process. HHG can image a molecular orbital and probe rotational and vibrational dynamics in the electronic ground state. Molecular bromine (Br2) serves as our exemplary molecule. Excitation at 400 nm transfers population from the X 1Σg+ ground state to the repulsive C 1Π1u state in which it dissociates (Fig. 9.8a). Dissociation is essentially adiabatic, resulting in two bromine atoms in the 2P3/2, |mJ| = 1/2 state. High-harmonic generation from a coherent superposition of two electronic states can proceed by ionizing from and recombining to the same or the other electronic state. All emitted fields are phaselocked to the generating field and interfere with each other, as illustrated in Fig. 9.8b. Once the overlap of the excited and ground-state vibrational wave packets is lost, however, the coherent superposition can no longer be distinguished from a mixed sample of distinct ground-state and excited-state molecules. Only harmonics created by ionizing and recombining to the same state are possible. Owing to the coherent nature of the attosecond high-harmonic pulse generation, the unexcited molecules act as local oscillators against which the dynamics are observed, so a transient grating technique can be used to reconstruct the amplitude and phase of emission from the excited molecules. A sinusoidal grating of excited molecules is formed using two pump beams that cross in the medium, as shown in Fig. 9.9a. Horizontal planes of excited molecules alternate with planes of 4 unexcited molecules. High harmonics are generated from this grating by a delayed 800-nm laser pulse (probe). Figure 9.9b and c shows the yields of harmonics 13 to 21 in both the zero-order (Fig. 9.9b) and the first-order diffraction (Fig. 9.9c). The intensity of the zero-order diffraction decreases in all harmonic orders following excitation and subsequently increases to a level that depends on the harmonic order. By contrast, the first-order diffraction signal (not present at negative times) increases, reaching its asymptotic value about 300 fs after the excitation pulse. The zero time-delay and a cross-correlation time of 50 fs is monitored through the appearance of even-order harmonics. Structural information can be extracted from the amplitude, which encodes the internuclear separation, by quantum interference at short times and by scattering of the recollision electron at longer times. The phase records the attosecond dynamics of the electrons, giving access to the evolving ionization potentials and the electronic structure of the transient molecule. A temporal shift of the high-harmonic field of less than an attosecond between the stretched and compressed geometry of weakly vibrationally excited Br2 in the electronic ground state could be documented. The ability to probe structural and electronic features, combined with high time resolution, make high-harmonic spectroscopy ideally suited to measuring coupled electronic and nuclear dynamics occurring in photochemical reactions and to characterizing the electronic structure of transition states. Take-home messages. The transient grating is one of the four-wave mixing techniques: the interference between two pump pulses creates a transient grating in the sample which diffracts another (probe) light beam(s). The intensity and phase conditions of the diffracted light (TG signal) reflect the dynamics and the optical and thermodynamic properties of the photochemically active species. Home works 1. What was the grating wavenumber in a thermal grating experiment with Evans blue if a lifetime of 80 μs was measured (see Fig. 9.5)? 2. What is the apparent lifetime of the TG signal if thermal and population gratings appear simultaneously? Estimate the lifetime of the observed TG after pulsed excitation of bacterial RC if the rate of charge recombination is (1 ms)-1 (the native ubiquinone10 is substituted by anthraquinone at QA). The conditions are the same as for thermal grating experiment with reference dye. 3. Discuss the Kramers-Kronig relationship. References Terazima M (2004) Time-Resolved Thermodynamic Properties of Intermediate Species during Photochemical Reactions. Bull. Chem. Soc. Jpn., 77, 23-41. Nagy L, Maróti P, Terazima M (2008) Spectrally silent light induced conformation change in photosynthetic reaction centers. FEBS Letters 582, 3657–3662. Wörner HJ, Bertrand JB, Kartashov DV, Corkum PB, Villeneuve DM (2010) Following a chemical reaction using high-harmonic interferometry. Nature 466, 604–607. 5 Fig. 9.1. The physical principal of the Transient Grating Spectroscopy (TGS). Fig. 9.2. Pulsed probe beam for TG. The ultrafast laser beam is split into three parts by two beam splitters: two “pump” beams (having wave vectors k1 and k2) and a “probe” beam (having wave vector kprobe). The two pump beams are spatially and temporally overlapped on the sample. If the pumping wavelength is resonant with an absorption wavelength, then the excitation of the mode is spatially modulated across the overlap region, resulting in a population grating. The weaker beam kprobe, spatially overlapped but time delayed, is then Bragg diffracted off this grating into a phase-matched signal direction: q =± (k1-k2)+kprobe. By scanning the time delay, t of the probe pulses with respect to the pump pulses, the amplitude of the population grating can be measured as a function of time. The grating decays as exp(-2t/τ) and thus the lifetime of the excited state (τ) can be measured. 6 Fig. 9.3. The excitation beam of the second harmonics of a Nd:YAG laser (λ = 532 nm, Δt = 10 ns) and a cw IR beam (YAG, 1064 nm) are split by a transmission grating (optical mask) and the first order diffracted beams are combined again on the sample using a concave mirror (f = 25 cm). In order to avoid overexcitation of the sample, the energy of the excitation beam is reduced by neutral density filters (NDF1, typically below 100 μJ). One of the cw IR beams is used for the probe beam of the TG signal. The other beam intensity is attenuated about 1/100 by a neutral density filter (NDF2) for the use of the local oscillation (LO) light and of the TG signal. The filter is slightly adjusted tilting by a computer and used for a fine adjustment of the phase of the LO field to the probe field by changing the optical path length inside. The LO light intensity is detected by a photodiode (PD1). The LO light intensity can be changed not only by the interference between the signal and the LO light, but also by the transient lens (TrL) signal created by the pump beam. The TG signal is obtained as the difference between minimum and maximum LO light intensities matching two opposite phases after filter adjustments. The spacing of the grating fringe (the grating wavenumber) is measured by the decay rate constant of the thermal grating signal from a calorimetric standard sample (bromocresol purple). The signal is averaged and stored by a digital oscilloscope. The absorption change induced by the pump beam is monitored by a He–Ne laser (590 nm) after the flash excitation (PD2). Fig. 9.4. The intensity of different components of the measured grating signal at different LO light conditions for QB reconstituted RCs. TG+ and TG- indicate the measured kinetics with two opposite phases of the LO light (Δφ = 180o and Δφ = 0o, respectively). TrL is the transient lens and TG is the transient grating as the difference of TG- and TG+. 7 Fig. 9.5. Temperature-dependence of the refractive index of water: lifetime and amplidude of the TG signal of the dye Evans blue. Fig. 9.6. The position of the primary quinone, QA at the loop formed by helical and random structures of the bacterial reaction center protein close to the membrane surface. The BPh in the active (A) electron transport branch and the characteristic amino acids around the QA (HisM219, AlaM260 and TrpM252) are also indicated. Picture was drawn by HyperChem using the crystal structure 1PCR downloaded from the Protein Data Bank, Brookhaven. 8 Fig. 9.7. Kinetics of observed TG signals after flash-induced charge separation in bacterial RC with fully (QB), half (QA) and not (no QA) reconstituted quinone acceptor side. Note that the decay kinetics follow that of the charge recombination in cases of QB and QA but the kinetics of the emptied quinone acceptor side is unique and reflects different property of the excited RC protein. Transition P-BPhA ↔ P+-BPhACharge separation + Charge recombination Time range 10 ns P-QA ↔ P+-QA- 100 ms P-QB ↔ P+-QB- 1s TG controlled by Kramers-Kronig relationship: d(OD) → dn (absorption change causes change of the refractive index) Solvent volume change: (dn/dV)·dV Protein relaxation ~ 40 μs Charge separated state → ground state (there is no absorption change but there is volume change dV due to expansion or compression of the molecule in charge separated state) Table. 9.1. TG signal induced by charge separation, recombination and accompanying conformational relaxation of the bacterial RC protein. The TG signal is determined by the Kramers-Kronig relationship and/or the volume change of the RC. 9 Fig. 9.8. High-harmonic interferometry of dissociating Br2. a, Potential energy curves of the ground and excited (C1Π1u) state of Br2 (black and blue lines, respectively) and the lowest electronic states of Br2+ (red lines) as a function of the internuclear separation R. Also shown (thicker lines) are the ground-state nuclear wavefunction (black) and the calculated excited-state wave packet (blue) at selected delays Δt following a 40-fs excitation pulse centered at 400 nm. b, Starting from the electronic ground state of Br2, a small fraction of the sample is excited and undergoes dissociation. An intense 30-fs 800-nm pulse (red) probes the dissociation dynamics at variable delays after excitation. The two electronic states, represented by their most weakly bound orbital, emit high harmonics (turquoise) that differ in amplitude and in phase (Δφ). From H. J. Wörner, J. B. Bertrand, D. V. Kartashov, P. B. Corkum and D. M. Villeneuve Nature 466, 604–607 (2010). 10 Fig. 9.9. High-harmonic transient grating spectroscopy. a, Two synchronized excitation pulses (400 nm) set up a transient grating of excitation in the molecular beam. A delayed 800-nm pulse generates high harmonics from the excited sample. The periodic modulation of the high-harmonic amplitude and phase in the near field (in the laser focus) results, in the far field (at the detector), in additional first-order diffraction (m = 1) signal. b, c, Evolution of the normalized intensities of harmonics 13 to 21 for parallel pump and probe polarizations in the zeroth order (b) and the firstorder diffraction side band (c) with pump–probe delay. All signals have been normalized to the signal in the zeroth order at negative time delays, corresponding to molecules in the ground electronic state only. From H. J. Wörner, J. B. Bertrand, D. V. Kartashov, P. B. Corkum and D. M. Villeneuve Nature 466, 604–607 (2010). 11 12