ELE_1462_sm_AppendixS1
advertisement

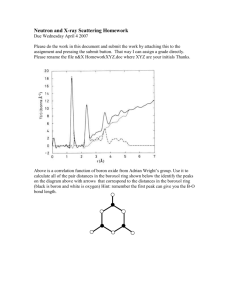
Supporting Information – Methods Bird Data. We used a global database on the breeding ranges of 9,626 extant, recognized bird species following a standard avian taxonomy (Sibley & Monroe 1990). We converted the species’ vector range maps into a gridded format using the equal-area Behrman projection, with a cell size of 96.486 km, equal to 1° longitude at 30° latitude, and conducted our analysis on the gridded data. A smaller grain size would permit analysis of range shapes at a finer resolution and reduce the overestimation in range size, particularly for species with linear distributions (Rahbek 2005). However, these concerns need to be weighed both against computational tractability of conducting simulations at this scale, and the accuracy of global species’ ranges (Hurlbert & Jetz 2007). A scale of around 1° is generally considered to be the finest resolution appropriate for global scale distribution data (Hurlbert & Jetz 2007). Using an equal area projection was necessary because of the need to conserve species’ range sizes in our cellular based simulations (Storch et al. 2006). Although the shapes and distances between cells in our grid are not constant, this distortion is only marked at very high latitudes where few species live (see Fig. S1 in Gaston et al. 2007). We discuss the implications of this for our measurement of shape below. Quantifying Range Shape. Previous studies investigating patterns in range shape have employed simple shape measures, such as N-S and E-W extent (Brown & Maurer 1989; Sandel 2009), but these fail to capture much of the detail in range configuration. The perimeter to area ratio has also been used to measure the shape of isodensity maps (Rapoport 1982; Ruggiero et al. 1998; Ruggiero 2001), a rough proxy for spatial patterns in range shape. Shape indices incorporating the perimeter are, however, highly sensitive to irregularities in the range boundary (Maceachren 1985; McGarigal & Marks 1995; Brown et al. 1996). As a result, indices based on maximum linear (l) and areal dimensions (a), are known to be more appropriate for quantifying shape circularity (Maceachren 1985). Of the various potential measures we calculated range shape (Rs) according to the following formula; Rs = l2/a (Fig S2) This is equivalent to the ratio of the long axis of a range to its average width (Graves 1988; Niemi et al. 1990). We selected this index for a number of reasons. First, the 1 index is scale invariant which was crucial given the enormous variation in range size amongst birds (Orme et al. 2006). Second, it produces an intuitive and easily conceptualised shape parameter i.e. a value of 3 indicates a shape 3x as long as it is wide. Finally, in contrast to other shape indices based on maximum linear dimensions (Clark & Gaile 1973; Maceachren 1985), it is linearly related to shape elongation. We note that although more complex shape indices, based on distances between all grid cells, exist (Maceachren 1985) these are not scale invariant and are difficult to standardise where the distances between cells vary geographically. To quantify the shape of the total gridded species range, we first calculated the shape of each of the constituent range fragments (McGarigal & Marks 1995) using graph theory, implemented in the “graph” and “RBGL” packages in R (http://cran.r-project.org/). Fragments were identified as clusters of occupied cells connected by cell edges; cells connected only by their vertices were assumed to be separate fragments (Fig. S2). To calculate the longest axis for each fragment, we used the shortest path distance through the range (i.e. passing through adjacent occupied cell centres) (Mcrae & Beier 2007) between all cells lying on the convex hull of the shape, and selected the longest of these (Fig. S2). This measure is preferable to a simple Euclidian distance (Graves 1988), because it accounts for concavity in range fragments (Mcrae & Beier 2007). In calculating the path length, the distances between adjacent cell centres were measured using great circle distances in the R package “fields” (http://cran.rproject.org/) (cells sharing edges or vertices were treated as adjacent). The use of great circle distances allowed the true distance over the Earths surface between cells to be calculated and so our measure of the range long axis was robust to the distortion of shape that occurs when the globe is viewed on the equal-area Behrman projection. In addition, a constant was added to the path length representing the distance from final cell centres to the actual external cell vertices. The overall range shape for a species was calculated as the average shape across fragments, weighted by fragment area (McGarigal & Marks 1995) (Fig. S2). Fragments smaller than 3 cells have a constant shape and were therefore not included in the calculation of range shape. 2 We calculated an alternative measure of range shape based on the scaled difference in the length of the eigen values of the variance-covariance matrix of distances between occupied cells (Maurer 1994). For full details of the general methodology we refer the reader to Maurer (1994). Here we briefly outline the details of how we applied this method to our global study. We first calculated the mean latitude and longitude of each range fragment and then calculated the great circle distance along the meridians and parallels from each occupied cell centre to these latitudinal and longitudinal averages. We then extracted the eigen values (e1 and e2) from the variance-covariance matrix of these internal range distances. Finally, the index of shape (Rs2) was calculated as the scaled difference between the length of the eigen values according to the following formula; Rs2 = (e1 - e2)/ (e1 + e2). Sources of environmental variables. Mean annual Temperature and Precipitation were obtained from New (2002) for the period 1961–1990 at 10 min resolution. Net Primary Productivity was obtained from Imhoff (2004). Mean elevation was calculated from 30-arcsecond resolution data developed by the USGS EROS Data Center available at http://eros.usgs.gov/products/ elevation/gtopo30.php). All environmental variables were resampled using the equal-area Behrman projection, with a cell size of 96.486 km to match the resolution of our species grid. SI References Brown J.H. & Maurer B.A. (1989). Macroecology - the division of food and space among species on continents. Science, 243, 1145-1150. Brown J.H., Stevens G.C. & Kaufman D.M. (1996). The geographic range: Size, shape, boundaries, and internal structure. Annu Rev Ecol Syst, 27, 597-623. Clark W.A.V. & Gaile G.L. (1973). The analysis and recognition of shapes. Geo Ann Ser B-Hum Geo, 55, 153-163. Gaston K.J., Davies R.G., Orme C.D.L., Olson V.A., Thomas G.H., Ding T.S., Rasmussen P.C., Lennon J.J., Bennett P.M., Owens I.P.F. & Blackburn T.M. (2007). Spatial turnover in the global avifauna. Proc Roy Soc B, 274, 1567-1574. Graves G.R. (1988). Linearity of geographic range and its possible effect on the population structure of Andean birds. The Auk, 105, 47-52. Hurlbert A.H. & Jetz W. (2007). Species richness, hotspots, and the scale dependence of range maps in ecology and conservation. Proc Natl Acad Sci USA, 104, 13384-13389. Imhoff M.L., Bounoua L., Ricketts T., Loucks C., Harriss R. & Lawrence W.T. (2004). Global patterns in human consumption of net primary production. Nature, 429, 870-873. Maceachren A.M. (1985). Compactness of geographic shape - comparison and evaluation of measures. Geo Ann Ser B-Hum Geo, 67, 53-67. 3 Maurer B.A. (1994). Geographical population analysis: tools for the analysis of biodiversity Blackwell Science, Cambridge, MA. McGarigal K. & Marks B.J. (1995). FRAGSTATS: spatial pattern analysis program for quantifying landscape structure. In: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station., p. 122. Mcrae B.H. & Beier P. (2007). Circuit theory predicts gene flow in plant and animal populations. Proc Natl Acad Sci USA, 104, 19885-19890. New M., Lister D., Hulme M. & Makin I. (2002). A high-resolution data set of surface climate over global land areas. Climate Res, 21, 1-25. Niemi R.G., Grofman B., Carlucci C. & Hofeller T. (1990). Measuring compactness and the role of a compactness standard in a test for partisan and racial gerrymandering. J Politics, 52, 1155-1181. Orme C.D.L., Davies R.G., Olson V.A., Thomas G.H., Ding T.S., Rasmussen P.C., Ridgely R.S., Stattersfield A.J., Bennett P.M., Owens I.P.F., Blackburn T.M. & Gaston K.J. (2006). Global patterns of geographic range size in birds. PLoS Biol, 4, 1276-1283. Rahbek C. (2005). The role of spatial scale and the perception of large-scale species-richness patterns. Ecol Lett, 8, 224-239. Rapoport E.H. (1982). Areography: Geographical strategies of species. Pergamon Press, New York. Ruggiero A. (2001). Size and shape of the geographical ranges of Andean passerine birds: spatial patterns in environmental resistance and anisotropy. J Biogeogr, 28, 12811294. Ruggiero A., Lawton J.H. & Blackburn T.M. (1998). The geographic ranges of mammalian species in South America: spatial patterns in environmental resistance and anisotropy. J Biogeogr, 25, 1093-1103. Sandel B. (2009). Geometric constraint model selection - an example with New World birds and mammals. Ecography, 32, 1001-1010. Sibley C.G. & Monroe B.L. (1990). Distribution and Taxonomy of the Birds of the World. Yale University Press, New Haven. Storch D., Davies R.G., Zajicek S., Orme C.D.L., Olson V., Thomas G.H., Ding T.S., Rasmussen P.C., Ridgely R.S., Bennett P.M., Blackburn T.M., Owens I.P.F. & Gaston K.J. (2006). Energy, range dynamics and global species richness patterns: reconciling mid-domain effects and environmental determinants of avian diversity. Ecol Lett, 9, 1308-1320. 4