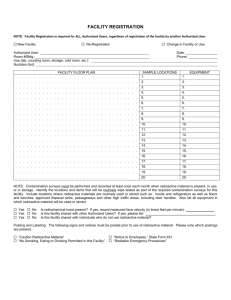
Importation of Radioactive Substances
advertisement

MINISTRY OF HEALTH STANDARDS & REGULATION DIVISION PHARMACEUTICAL & REGULATORY AFFAIRS DEPARTMENT JAMAICA, WEST INDIES Importation of Radioactive Substances Product Particulars: 1. NAME OF RADIOACTIVE SUBSTANCE: .…………………………………………………………………………………………….……….. 2. NAME AND ADDRESS OF MANUFACTURER/SUPPLIER:.…………………………… ……………………………………………………………………………………………………… 3. NAME AND ADDRESS OF APPLICANT:…………………………………………………. ……………………………………………………………………………………………………… LIST OF REQUIREMENTS FOR ASSESSMENT PURPOSES: An indication of the purpose for which the product is being imported. A formulation statement (list of the chemical constituents in each product and the concentration of each constituent). Details of the radioactive substance should be provided. Technical product information which should include: - product literature/manual - applications of the product - chemical properties - occupational routes of exposure - physical and chemical dangers - exposure risks [short-term and long-term] - environmental risks Storage and handling requirements Precautionary measures to ensure safety Emergency procedures for handling spillage Responsibility of the manufacturer/supplier Details of the shipping process and safety measures An undertaking from the manufacturer/supplier stating that the radioactive substance will be retrieved and returned to the country of manufacture/origin for disposal, once the life of the radioactive substance has been exceeded. Information on the local expert in radioactive technology who will be responsible for handling the product. This should include the name, contact details and qualifications. Revised November 2012