Pharmacy Advisor
advertisement

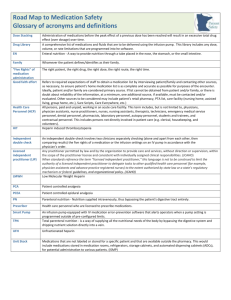
THE PHARMACY ADVISOR A Publication of the Beth Israel Deaconess Medical Center Department of Pharmacy and Pharmacy & Therapeutics Committee Volume II, Issue 2 Medication Safety “ It’s Time For Standards To Improve Safety With Electronic Communication Of Medication Orders” The above title is taken from a feature article in a recent medication safety alert from the Institute For Safe Medication Practices (ISMP). This timely communication coincides with the medication safety initiatives that have been underway for some time at BIDMC as we have constructed our Provider Order Entry and Pharmacy computer systems. The article states that, “Among its many benefits, electronic communication of medication orders allows for more accurate conveyance of information than handwritten formats. However, if the conventions used to communicate electronic information are not considered carefully, computerized order processing actually may contribute to medication errors.” It is further communicated that, “To avoid problems, healthcare technology vendors need a set of accepted standards for the electronic communication of medication information.” Outlined in the safety alert are preliminary guidelines intended to serve as a starting point for standards in the way prescribed medications are presented and communicated in electronic formats. Excerpts from the guidelines are included later in this article. The complete medication safety alert and guidelines can be found on the ISMP web site at http: www.ismp.org. The authors recognize that these initial guidelines are incomplete and certain portions are expected to attract discussion about their benefits and limitations. Individual practitioners and institutions are encouraged to share comments and suggestions on this very important issue. Please send comments to ismpinfo@ismp.org. Continued on page 2 INSIDE THIS ISSUE 1 2 3 Medication Safety With Electronic Communication Of Medication Orders Drug Shortage Management: IV Pantoprazole (Protonix®) Formulary Update: Ziprasidone (Geodon®) 4 Kinetics Corner: Antimicrobials: When Pharmacokinetics meets Pharmacodynamics 5 BIDMC Guidelines for Anticoagulation in Patients with Heparin Induced Thrombocytopenia The Pharmacy Advisor is a publication of the Department Of Pharmacy and the Pharmacy & Therapeutics Committee at the Beth Israel Deaconess Medical Center, Boston, MA 02215 Writing/Editorial Board: Katherine Giampietro, PharmD Christopher McCoy, PharmD Diane Soulliard, PharmD Bruce Bistrian, MD, Co-Chair P&T James Heffernan, MD, Co-Chair P&T Francis P. Mitrano, M.S., RPh February 2003 Drug Shortage Management: IV Pantoprazole (Protonix®) A nation-wide drug shortage of parenteral pantoprazole has affected product availability at BIDMC and elsewhere for several months. The shortage is attributed to increased demand and supply constraints and is expected to continue through mid-2003. Until the shortage is resolved, the manufacturer, Wyeth-Ayerst, communicates that product availability will be on an allocation basis and institutions will be limited to a 50% monthly allocation of previous purchases. To ensure that patient-care needs can be met with the limited allocation of parenteral pantoprazole, the BIDMC will enforce the following Proton Pump Inhibitor (PPI) utilization guidelines: Oral administration of pantoprazole is the preferred route of therapy for patients requiring acid blockade with a PPI and are able to tolerate oral medications. Pantoprazole delayed–release tablets should not be crushed and therefore, the preferred PPI for patients requiring therapy via the nasogastric or enteral route is lansoprazole suspension. Such patients requiring PPI administration will receive a bicarbonate-based simplified lansoprazole suspension (SLS) prepared by pharmacy. Patients who require acid blockade and are unable to receive therapy (PPI or H2 receptor antagonist) via the oral or enteral route should receive a parenteral H2 receptor antagonist (IV famotidine) as the preferred parenteral agent of choice unless it has been shown to be ineffective or contraindicated. Parenteral pantoprazole (Protonix) will be reserved for those patients unable to receive therapy via the oral or enteral route and for whom parenteral famotidine is ineffective or contraindicated. More specifically, use of parenteral pantoprazole will be limited to those patients who meet the above conditions and have one or more of the indications listed below: Patients with severe erosive GERD: Dose: Pantoprazole 40mg IV QD Patients with hypersecretory disease (i.e. Zollinger Ellison Syndrome): Dose: Pantoprazole 80mg IV Q12H (some patients may require higher doses) Patients with an active GI bleed with an erosive source (i.e. ulcers or severe erosive esophagitis) Pantoprazole 80mg IV load, followed by 8mg/hour Recommendation by GI Consult There is no literature to suggest that use of an intravenous PPI is superior to conventional agents used for stress ulcer prophylaxis, such as the H2-receptor antagonists. Accordingly, patients requiring a parenteral agent for this indication should receive IV famotidine as the first-line agent of choice. The pharmacy will continue to provide updates regarding the IV pantoprazole shortage. P&T Formulary Update Medication Safety: continued from page 1 Ziprasidone added to the inpatient BIDMC formulary Below are excerpts from the ISMP Draft Guidelines For Safe Electronic Communication of Medication Orders. Medication safety has been at the forefront of the POE and Pharmacy system development at BIDMC and many of the recommended guidelines have already been incorporated into our electronic systems. Safe presentation of drug nomenclature and dose expressions in electronic systems: Ziprasidone (Geodon ) is an atypical antipsychotic agent. FDA Approved Indications: Ziprasidone is indicated for the treatment of schizophrenia. Ziprasidone intramuscular (IM) is indicated for the treatment of acute agitation in schizophrenic patients for whom ziprasidone treatment is appropriate and IM antipsychotic medication is required for rapid agitation control. Pharmacokinetics: Ziprasidone is well absorbed orally, reaching peak plasma concentrations in 6-8 hours. Bioavailability is approximately 60% and is increased up to two-fold in the presence of food. Advanced age, sex, and race do not appear to influence kinetics to a significant extent and dosage modification for age or gender are therefore, not recommended. The bioavailability of intramuscular ziprasidone is 100%. After administration of single IM doses, peak serum concentrations typically occur at approximately 60 minutes post-dose or earlier and the mean half-life ranges from two to five hours. Exposure increases in a dose-related manner and following three consecutive days of IM dosing, little accumulation is observed. Contraindications, Warnings, And Precautions: Ziprasidone has been associated with dose-related prolongation of the QT interval. Because of this and the consequent danger of life-threatening arrhythmias (e.g. torsade de pointe and sudden death), its use is contraindicated in patients with a known history of QT prolongation, cardiac arrhythmias, recent acute myocardial infarction, or decompensated heart failure. Concomitant administration of drugs that interfere with ziprasidone metabolism, drugs known to prolong the QT interval, or drugs likely to cause electrolyte imbalance should be avoided. Baseline serum potassium and magnesium screening should be performed and continually monitored during therapy. Adverse Reactions: Side effects in clinical trials have included dyspepsia, constipation, nausea, abdominal pain, dry mouth, sedation, headache, postural hypotension, dizziness, insomnia, akathisia, and rash. See package insert for the full listing. Dosing: The initial daily dosing of ziprasidone is 20 mg BID with food. When required, dose adjustment should be made at intervals of not less than 2 days to a recommended maximum dose of 80mg bid. The recommended dose of IM ziprasidone is 10 to 20 mg up to a maximum of 40 mg per day. Therapy with IM ziprasidone for more than 3 consecutive days has not been studied and oral ziprasidone should replace IM administration as soon as possible if continued therapy is required. Conclusion: Ziprasidone is an effective antipsychotic with efficacy comparable to haloperidol. It has not been associated with weight gain, making it unique in this class of pharmacologic agents. Ziprasidone has been associated with increases in QTc interval, which will restrict its use in some populations and may make it a second-line agent. The IM product offers an alternate choice to current therapies and based on preliminary evaluation, appears as effective as haloperidol in the management of acute psychosis and better tolerated with regard to sedation and movement disorders. BIDMC Restrictions: Parenteral ziprasidone will be reserved for use in the emergency department, psychiatric emergencies (Code Purple) and in-patient psychiatry, when intramuscular antipsychotic medication is required for rapid agitation control. List all products by generic name as the primary drug nomenclature. Do not include the salt of the chemical when expressing a generic name unless there are multiple salts available. As appropriate, list brand names in a requisite field. Express suffixes that are part of the brand name (e.g., SR, SA) within both the generic name and the brand name field. Avoid use of all potentially dangerous abbreviations and dose expressions including the following: a. Do not use trailing zeros (5 mg, never 5.0 mg). b. Use leading zeros for doses less than one measurement unit (0.3 mg, never .3 mg). c. Spell out the word UNITS. d. Express weights and measures in a standard fashion and use USP standard abbreviations for dosage units To avoid confusion, do not abbreviate drug names. Design features for electronic order entry systems (pharmacy and prescriber) that support safe communication of orders: Provide adequate space for items in data fields used to communicate drug names, dosing units, routes of administration, and frequencies. Provide a field that requires entry of the purpose for the following types of medication orders: all prn medications. Provide the ability to clearly communicate medications prescribed for specific, non-routine administration times or under certain conditions (e.g., with dialysis, while NPO, until tolerating liquids, prior to surgery). Provide the capability to link medications only to the appropriate routes of administration available for each drug. Provide a mechanism to facilitate safe order entry of complex medication regimens (e.g., chemotherapy, sliding scale insulin) and medications that require a tapering dosing schedule (e.g., steroids) so that the orders appear clearly and in a logical order for those who dispense and administer the medications. Provide the capability for clinicians to select the time or date to begin drug administration, regardless of when the order was entered. Provide a mechanism to place medication orders on hold under specified conditions, and to alert users at specified times while a medication is on hold. To prevent the risk of misinterpretation, limit the potential for free-text entries in CPOE systems. The complete guidelines and additional topics for electronic medication safety standards are included in the full text ISMP article. Should you have any comments or suggestions regarding medication safety in the BIDMC POE system, please forward them to the Department of Pharmacy. Kinetics Corner Antimicrobials: when pharmacokinetics meet pharmacodynamics Optimization of antimicrobial therapy includes consideration of both the pharmacokinetic and pharmacodynamic properties of the antimicrobial agent selected for use. An overview is presented here to provide a general understanding of these principles as they relate to antimicrobial selection and utilization. Pharmacokinetics is the study of the time-dependent evolution of drug concentrations and distribution within various body compartments, often through use of differential equation models. More specifically, the pharmacokinetics of a medication rests in the absorption, distribution, metabolism and elimination of the drug and its relationship to efficacy and safety. Pharmacodynamics refers to the effectiveness of a medication as a function of time-dependent concentrations. More specifically, the pharmacodynamics of an antimicrobial agent rests in the activity of the drug at the site of action, ordinarily on the outer surface (e.g., penicillin binding proteins) or inner structures (e.g., ribosomes) of the microbial pathogen. Antibiotics are classified as demonstrating optimal pharmacodynamic activity depending upon the amount of time they are exposed to the site of action (time dependence) or the ability to “supersaturate” the site of action at a high concentration (concentration dependence) for a short period of time. For every organism, there is a minimum concentration of antimicrobial above which, there is consistent inhibition of growth and/or replication and a trend toward decreased bacterial resistance. This is termed the minimum inhibitory concentration (MIC). Concentration dependent antimicrobials have been demonstrated to achieve optimal antimicrobial activity when the ratio of the antibiotic peak (Cmax) to the MIC, also known as the peak to MIC ratio, is highest without reaching a toxic range. Generally, acceptable ratios are 8 to 10 times the MIC for that agent. Hence, there has been a trend toward the application of supratherapeutic doses at an extended interval for antibiotics that fall within this category (e.g., extended interval aminoglycosides). Other models have demonstrated the benefit of measuring a 24-hour area under the curve (AUC), which is the total concentration of antibiotic over a 24h period and its relationship to the MIC (termed the AUC to MIC ratio.) The goal AUC/MIC ratio varies dependent on the organism and is generally higher for gram-negative organisms, about 100, versus gram-positive organisms, including Streptococcus pneumoniae, about 35. Examples of concentration dependent antimicrobials include the aminoglycosides (e.g., tobramycin, gentamicin and amikacin) and the fluoroquinolones (e.g., ciprofloxacin and levofloxacin). The translation for drug monitoring of concentration dependent antibiotics in practice is that a peak level drawn on an extended interval high dose regimen of gentamicin will result in a level that far exceeds the “standard” peak levels reported by the laboratory, e.g., 6-10 mcg/ml. This may alarm some, but the intent of the high dose is to “overwhelm” the microbial site of action. This practice has not been associated with increased toxicity as the time of the peak concentration is short, and the nephrotoxic effects of aminoglycosides, are related to the saturation of the renal tubules over prolonged periods. This provides the rationale for not repeating a 5mg/kg dose at the standard q8hourly interval. Active transport mechanisms are allowed to facilitate drug elimination from the tubules when true trough levels (within 30 minutes of the next dose at steady state) remain below 1 or 2. Additional data supports the relationship of optimizing the 24-hour AUC/MIC ratio in acutely ill patients. Thomas, et.al. (Antimic Agents Chemother 1998;42:521-7) demonstrated that a 24-hour AUC/MIC ratio greater than or equal to 100 was associated with a reduced risk of emergence of resistance, 9% vs. 82%. In some cases, this AUC/MIC ratio was measured when therapy had included both a beta lactam and fluoroquinolone antibiotic to target a single pathogen, a method of measurement that has not been well translated in vitro to in vivo. Additionally, this AUC/MIC ratio and its relationship to resistance was not reproducible among patients treated with beta lactam antibiotics alone. Only the patients treated with ciprofloxacin demonstrated a statistically significant difference lending more evidence that a higher total concentration for fluoroquinolones may be associated with less resistance. This does not imply that resistance will not occur with fluoroquinolone antibiotics, but rather that certain dosing strategies with fluoroquinolones can optimize outcomes. Time dependent antimicrobials achieve optimal antimicrobial activity when the steady state trough concentrations remains above the MIC for at least 50% of the treatment course and the AUC remains at an appropriate level. Models of continuous infusions or longer infusion times of larger doses have been investigated. Examples of time dependent antimicrobials include the beta-lactam antibiotics (e.g., penicillin, ampicillin, ceftazidime, cefepime, meropenem), which demonstrate optimal killing with a prolonged time above MIC and no killing effect below the MIC. Other drugs that are deemed time-dependent are the macrolide antibiotics (e.g., azithromycin, erythromycin and clarithromycin), vancomycin, clindamycin and linezolid, which demonstrate optimal killing with a prolonged time above MIC and a killing effect below the MIC. The translation for drug monitoring of time-dependent antibiotics in practice is that the trough level should remain above the MIC for at least half of the 24-hour period. For example, the goal trough levels for vancomycin are 5 to 15mcg/ml, above a general MIC of 2mcg/ml. Peak levels are not necessary for vancomycin as the ratio of peak/MIC is not as important for time dependent antimicrobials. The clinical significance and application of these theories is variable. Drug levels are not routinely drawn nor recommended for betalactam or fluoroquinolone antibiotics. However, drug levels are often ordered for aminoglycosides and vancomycin. The usefulness of these levels lies in the appropriateness of timing of serum samples relative to dose administration and their interpretation. Common errors include drawing levels too often or at less than optimal times and adjusting dose or schedule without consideration of these factors. The intent of antimicrobial therapeutic drug monitoring is focused on optimizing activity while preventing drug related toxicities. This includes the monitoring and consideration of objective measures of efficacy, (e.g., WBC count, temperature, sputum production) and measures of toxicity, (e.g., BUN/Cr, urine output, ataxia). Laboratory based pharmacokinetic models help to elucidate more predictable and improved general practice models without the need for drug levels for every patient on a daily basis. Important pharmacokinetic concepts to consider include the premise that in patients with varying degrees of renal deficiency, extension of the drug interval at a higher dose may be more appropriate for concentration dependent antimicrobials, aminoglycosides and fluoroquinolones, e.g., gentamicin 6mg/kg q48h or levofloxacin 500 mg q48h. Contrarily, reduction in the dose of a drug may be more appropriate for time dependent antimicrobials, e.g., cefazolin 1gram IV q8h versus 2 g IV q8h. For assistance in the interpretation of antimicrobial levels, dosing and/or scheduling of antimicrobials, please call your unit based pharmacist, or Christopher McCoy, PharmD, pager 39392. BIDMC Guidelines for Anticoagulation in Heparin-Induced Thrombocytopenia Heparin-induced thrombocytopenia (HIT) is a serious complication associated with heparin therapy and occurs independent of the route of administration, indication and dose of heparin. Two distinct types of HIT, Type I and Type II, are recognized and involve different causal mechanisms. The more serious, immunologic form (HIT Type II), is characterized by the formation of antibodies against the heparin-platelet-factor 4 (PF4) complex with an acute drop in platelets (often dropping to 60,000/µL or below) and generally occurs 5 to 14 days following initiation of heparin (earlier, if the patient has been previously exposed to heparin.) The second, non-immunologic form (HIT Type I) occurs in 10-20% of patients and is characterized by a decrease in platelet count (usually not less than 100,000/µL) occurring 1-2 days following initiation of heparin. Type I HIT often resolves on its own, despite continued heparin therapy, without significant clinical sequelae If HIT type II is suspected, all heparin products must be discontinued and the patient evaluated for the need of further anticoagulation. If anticoagulation is required, a PF-4 test may be requested and alternate anticoagulant agents selected. Low molecular weight heparins are not appropriate alternatives due to the high risk of cross-reactivity to HIT antibodies. Direct thrombin inhibitors (DTI) are newer anticoagulants that inactivate both soluble and clot-bound thrombin and do not cross-react with the HIT antibody. Available DTI’s include argatroban, bivalirudin and lepirudin. All three are administered by continuous infusion and require monitoring of aPTT or ACT. Argatroban and lepirudin have been shown to reduce the risk of death, amputation and new thromboembolic complications and are both FDA approved for use in patients with HIT. Argatroban is preferred to lepirudin in HIT patients [not undergoing percutaneous coronary intervention (PCI) or catheterization] since 40% of patients receiving lepirudin develop antibodies on first exposure and are at risk of anaphylaxis upon re-exposure. Bivalirudin is FDA approved for use in patients undergoing PCI or cardiac catheterization and can also be used as an alternative to argatroban or lepirudin for patients with HIT not undergoing PCI. Use of argatroban in patients with moderate to severe hepatic impairment (Child-Pugh score >6) should be avoided as the drug is hepatically metabolized. Lepirudin and bivalirudin are renally eliminated and require adjustment for renal impairment (estimated CrCl < 60 ml/minute). Extreme caution is required in dosing lepirudin in patients with a CrCl 15 ml/minute and it should be avoided in patients undergoing hemodialysis. Guideline for Management of Heparin-Induced Thrombocytopenia (HIT) HIT suspected in patient receiving heparin in setting of platelet count drop 50% below a pre-heparin platelet count value, and/or with clinical signs & symptoms of heparin-induced thrombocytopenia with thrombosis (HITTS) STOP ALL HEPARIN AND HEPARIN FLUSHES Request PF4-heparin antibody test if patient requires anticoagulation PF4 Positive - Stop all heparin including flushes; Choose alternative Antithrombotic if patient requires anticoagulation PF4 Negative - Clinical Decision Making; Consider alternative Antithrombotic if HIT likely and patient requires anticoagulation HIT patients requiring anticoagulation and NOT undergoing PCI/Cath and NOT with Acute Coronary Syndrome Moderate to Severe Hepatic Impairment NO ARGATROBAN (Preferred Direct Thrombin Inhibitor in Non-PCI/Cath patients) HIT/HITTS: 2 mcg/kg/min by continuous infusion with no bolus dose Monitor aPTT to goal and follow INR closely if on warfarin therapy Dosing adjustment required in hepatic impairment. (0.5 mcg/kg/min) YES LEPIRUDIN¶ HIT/HITTS: 0.4 mg/kg bolus followed by 0.15 mg/kg/hr infusion* Monitor aPTT to goal ¶ Anaphylaxis can occur on reexposure to lepirudin due to antibody formation *If estimated creatinine clearance < 60 ml/min must adjust Lepirudin dose or consider Argatroban. Consult a pharmacist for assistance in dosing HIT patients undergoing PCI/CATH or with Acute Coronary Syndrome (ACS) BIVALIRUDIN* PCI dose: ADMINISTER IN CATH LAB ONLY 0.75 mg/kg bolus then 1.75 mg/kg/hr during the procedure; Obtain ACT 5 minutes into infusion; If < 225 give one 0.3 mg/kg bolus Monitor ACT to 225 ACS dose: 0.2 mg/kg/hr Monitor ACT to goal * If estimated creatinine clearance < 60 ml/min must adjust bivalirudin dose when continuing therapy beyond the cath lab procedure or when using for ACS or DVT. Consult a Pharmacist for assistance in dosing Child-Pugh classification of severity of liver disease considers degree of ascites, serum bilirubin and albumin, prothrombin time and degree of encephalopathy. The PF4 test assays for the presence of antibodies directed against platelet factor 4 (PF4)-heparin complexes, which can result in thrombocytopenia, platelet activation/aggregation, and prothrombotic complications. The Pharmacy Advisor 4