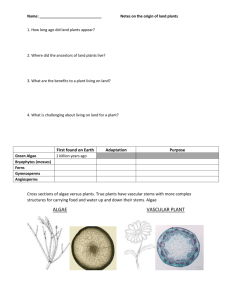
Lymph node biopsy
advertisement

RESIDENT/PA GROSS PATHOLOGY SHADOWING MANUAL Revised June 2011 1 Table of Contents: Resident/PA Shadowing Program Page Directory Diagrams Histology Laboratory Room 1 Room 2 AP Philosophy Weekly Histology Quality Dashboard LEAN PowerPoint AP Redesign PowerPoint Safety Safety Tips Patient Safety PowerPoint General Policies Interaction of team (don’t go solo) – for questions, always go to team members Electronic Calendar Placement of Requisition Forms Gross Conference Cases Gross Cutting Manual Grossing Station Clean-up Protocol / Supplies Important Dictation Tips/Instructions Frozen Section Dictation Examples of Dictation General Specimen Handling General Inking Guidelines Cutting Techniques / Thorough Gross Sectioning Common Tips for Specimen Orientation Gross Only’s Completed Specimen Placement o Diagram of Storage Cart Paperwork How to Complete a Surgical Pathology Log Sheet for Cassettes Example Log sheets for Blocks Special Procedures Decal Procedures Tabletop Band Saw Instructions Digital Camera Instructions Tissue Procurement Special Case Types Lymphomas Placentas Histology Lab Histology Procedures Case Filing and Retrieval Goals for Specific Specimen Types Evaluation Exercise 2 2 3 4 5 6 PP PP 7-9 PP 10 10 10 10 10 11 12-15 12 16-19 20 21 22 23 24 25 26 27-28 30-33 34 35 36 378-38 39-41 42-43 44 45-46 47-4 DIRECTORY NAME Lisa Taulbee Mary Jo Bishop Debbie Woodard Amy Wilcik Paulette Dozier Rebecca Heberlein-Rollins Tina Fields Matthew Gabbeart Millie Holley Tiffany Vail Donna Renner-Chuey Moragh Goyette Angie Florn Deb Postiff TITLE Supervisor, Histology Lab Lab tech, Room 2 (Accessioning) Lab Tech, Room 1 (Accessioning) Lab Tech, CVC Office Manager, Surgical Pathology Slide Librarian Senior Histotechnologist, IPOX Pathology Assistant Pathology Assistant Pathology Assistant Pathology Assistant Lab Tech, Room 2 Procurement Tech Procurement Tech UH Faculty Administrative Assistants Beth Minors, Executive Assistant 936-1888 Dr. Jeffrey Myers, Director, Anatomic Pathology Christine Betts, Administrative Assistant 647-9125 Dr. Priya Kunju, Director, Genitourinary Pathology Dr. Thomas Giordano Dr. Celina Kleer Dr. Peter Lucas Dr. Jon McHugh Dr. Judy Pang Dr. Angela Wu Mary Currie, Administrative Assistant 936-6776 Dr. Robertson Davenport, Director, Blood Bank & Apheresis Dr. Laura Cooling Dr. Raja Rabah, Director, Pediatric Pathology Dr. Lindsay Schmidt Liz Schuiling, Administrative Assistant 936-6775 Dr. Claire Michael, Director, Cytopathology Dr. Xin Jing Dr. Stewart Knoepp Dr. Michael Roh Laurie Quay, Administrative Assistant 232-0022 Dr. David Lucas, Director of Surgical Pathology 3 NUMBER 615-8989 / lab 6-6818 762-6092 647-6273 762-5910 936-6801 615-3401 763-4529 Pager # 31034 Pager # 32433 Pager # 8340 Pager # 8183 936-6799 764-8025 or 936-8099 Pager # 8952 764-8025 or 936-8099 Pager # 8952 Histology Lab Processors Slide Reconciliation Counter Slide Library IPOX Slide Pick-up and Distribution Sr. Histotechs Staining Special Stains Embedding Front Desk Sink Microtomes Re-Cut/Stain Requests Office Room 2 4 Computer Blocks Calex Vial RPMI Fridge Cassette Labeler ROOM 1 Table Top Saw GI Grossing Station GU CART Specimen Vented storage #2 Storage Cart Gross Conference Cases Tissue Procurement Waste Formalin Cytogenetics Requisitions Room 1 Grossing Station Formalin TPC Supply Cabinet Snap Freeze / Mold Foil Sink (clean) Scale IF Accessioned Specimens OR Log GA Accessioning Sign out / Reading Room 5 Flow Container / Vial Frozen section stainer Counter O.C.T. Flammables Storage #1 Sink Cryostat #2 Frozen Section Grossing Station Cryostat #1 Specimen Vented GU Grossing Station Specimen Storage Trash Gross Conference Cases BE Grossing Station Accessioning Station 6 Window Accessioning Station Cart Flammables Fax Trash Histotech Grossing Station ROOM 2 Specimen Storage Vented Cabinet #2 GS Grossing Station GS Cart BE Cart Clean Sink Vented Cabinet #1 Scale B5 Counter HISTOLOGY WEEKLY QUALITY DASHBOARD Inclusive dates: 5/5/08 thru 5/9/08 1. Volume and TAT Histology Total cases Total blocks % On time BE 59 511 96.6 Frozen Sections GA 483 1271 100 GS 155 694 94.8 GU 67 729 97.0 HP 79 285 98.7 ID 271 940 100 IF 163 1029 99.4 IS 78 121 100 MD 145 287 93.8 AUT 11 129 100 CVC Room1 Special Stains HC IPOX # Total Frozen Sections 5 141 # Total Requested 224 991 % On Time (<20 min) Single-part Cases Only 60 93 % <24 hr TAT 100 100 Slide Librarian Conference Diagnosis In-House Send Out # Total Requests 26 26 6 10 # Total Cases 100 36 7 17 # Same Day (TAT) 23 23 5 16 % Within 24 Hours (TAT) 100 100 100 100 # of overdue Research requests* (# of total cases within those requests): 0 (0) * overdue meaning > 2 weeks since requestors submission date 2. Patient Safety # Distributed mislabeled cases 0 # Lost specimens # Histology processing errors # Specimen/requisition deficiencies 0 1 10 OTHER 119 323 NA Totals 68 160 67 100 Frozen section vs. permanent diagnosis discrepancies Inclusive dates: 4/28/2008 thru 5/2/2008 # Total Frozen Sections 125 # Minor Discrepancies (% of Total) 3 (2.4) # Major Discrepancies (% of Total) 1 (0.8) 3. Processing efficiency and quality % excellent H and E stain: 100 (n=20/week of a random selection of slides) # reprocessed cases: 2 case(s) totaling 9 block(s) # AM unprocessed blocks: 9 case(s) totaling 55 block(s) # AM unaccessioned cases: 187 # ungrossed accessioned cases: 115 # Special stains reprocessed: HC: 0 TOTALS 1630 6315 98.5 IHC: 0 4. Customer Service Example of the Week This week we recognize the “morning crew” of Histology: Kristen Bond, Stephanie Drewery, Judith Dunn, Meagan Hillman, Sharon Kelly, Theresa Pace, April Marr, and Kathy Williams. While we are fast asleep, these dedicated professionals arrive in the middle of the night to start processing the day’s workload. As a team, they effectively prioritize and orchestrate variable daily workloads to achieve expected goals in providing timely, quality patient service. Together, they exemplify the Michigan Difference that has moved this lab to an environment of continuous improvement! 7 UMHHC PATHOLOGY GROSSING SAFETY PROCEDURES Body Substance Precautions (BSP) Body substance precautions are observed throughout the laboratory to prevent contact with blood and other potentially infectious materials. All body fluids are considered potentially infectious. All procedures involving blood or other moist body substances shall be performed in such a manner as to minimize splashing, spraying, spattering, and generation of droplets of these substances. Blood and other body fluid spills should first be soaked up with absorbent material (e.g., paper towels). The area should then be cleaned using 10% bleach solution. Work surfaces/laboratory bench tops shall be cleaned and decontaminated after contact with blood or other potentially infectious materials or after completion of laboratory procedures. Personal Protective Equipment (PPE) Personal protective equipment (PPE) will appropriately be utilized in all laboratories as assigned based on the task assessment, which does not allow blood, other potentially infectious materials, or hazardous chemical exposure to pass through to or otherwise reach the employee’s work clothes (e.g., scrubs), street clothes, undergarments, skin, eyes, mouth, or other mucous membranes under normal conditions of use and for the duration of time the protective equipment is used. All PPE is removed immediately prior to leaving the work area, or as soon as possible, and placed in an appropriately designated area or container for storage, washing, decontamination, or disposal within each individual laboratory. Gloves must be worn for blood and body fluid precautions and when working with heat sources, subzero cold sources, and hazardous chemicals. Gloves must be removed when outside the technical area. Gloves must be replaced whenever torn or appreciably soiled with blood or body fluids. Hands must be washed immediately after removing gloves. When removing gloves, grasp the cuff of the glove and pull the glove off inside out. Avoid touching the skin. Never wash and reuse disposable gloves. Disposal – if not visibly contaminated dispose in regular trash. If visibly grossly contaminated dispose in biohazard receptacle. Personal Protective Equipment Lab coat – knee length, long sleeved, buttoned Gloves – nitrile, chemical resistant Safety glasses – must meet ANSI Z87.1 Task Tasks that involve exposure to potential biological or chemical hazards Tasks that involve exposure to potential biological or chemical hazards Tasks that involve exposure to potential biological or chemical hazards 8 UMHHC PATHOLOGY GROSSING SAFETY PROCEDURES (cont) Hazardous Chemicals A system of local and/or general exhaust is recommended to keep employee exposures below the established exposure limits. Local exhaust ventilation is generally preferred because it can control the emissions of the contaminant at its source, preventing dispersion of it into the general work area. Use center of cutting board where optimal exhaust control is provided. Place specimen / cups on or near bench top away from breathing zone. Cap open containers and waste funnel to minimize vapors. Use magnifying tools on Mopec grossing station to prevent bending into direct vapors. Store fixed specimens and blocks in vented storage cabinet. Wear impervious protective clothing, including boots, gloves, lab coat, apron or coveralls, as appropriate, to prevent skin contact. Use chemical safety goggles and/or a full face shield where splashing is possible. Maintain eye wash fountain and quick-drench facilities in work area. Accidental Release Measures: Ventilate area of leak or spill. Wear appropriate personal protective equipment. Isolate hazard area. Keep unnecessary and unprotected personnel from entering. Contain and recover hazardous material when possible. Collect waste in an appropriate container or absorb with an inert material (e. g., vermiculite, dry sand, earth), and place in a chemical waste container in accordance to UMHHC waste management policies. For large spills with excessive fumes, contact UMHHC emergency response team. Do not carry a specimen container without a lid if there is fixative in the container. If you drop the container or drip any amount of fixative on the floor, immediately use spill absorbent and paper towels to clean it. Do not walk about the room with a specimen dripping in your hand; use a paper towel, a lid or a surgical towel. Keep the lids of the inks on the bottles as much as possible. If ink spills on the workstation or on the floor, wipe it immediately, if possible. If any fluid is allowed to dry it is much harder to clean, so try to keep the work surface wet. Radiation Safety Radioactive sources can include liquids or solids that release radiation above normal background levels. Solids are most likely to be encountered in prostate specimens. See Pathology policy (found on the pathology home page) on handling prostate seeds. Seeds must not be handled until cleared by Safety Management Services. Sharps Do not walk around the room carrying sharp objects. If you must, be sure to check for anyone in your path and announce that you will be walking with a sharp object. Keep the sharp edge facing the floor; carry it below your waist and the tip pointed to the floor. Keep the blade handle firm in your grip and no more than 2 inches from the side of your leg. 9 Needles, syringes, disposable razor blades, and other sharp items shall be placed in an UMHHC-approved puncture resistant container for disposal. UMHHC PATHOLOGY GROSSING SAFETY PROCEDURES (cont) Used needles must not be cut, bent, broken or recapped by hand before disposal due to increased chance for injury when needles are manipulated. Sharps containers shall be located in all areas where needles and sharps are used and shall be secured so they will not be knocked over and their contents spilled. Grossing Tools: The #22 blade goes on the red-brown handle and the #60 blade fits the gray handle. To put the #22 or #60 blade on their handle, put the tip of the blade on the cutting board and gently push down on the handle until it clicks into the opening. Keep your hand at a safe position to the side and do not hold the blade in your hand. Do not point the tip of the blade toward your hand or pointed at anyone. To use the blade remover: Carefully slide the tip of the blade into the opening for the blade remover. Press down on the removal device closest to the handle (this will lift the back edge of the blade off of the holder). While still pressing on the removal device, slide the blade and device off of the handle. Put the blade over the sharps container and let it drop into the container. To remove a blade from the handle using forceps. Point the tip of the blade at the back of the workstation and keep it low to the cutting surface. Keeping your fingers close to the tip of the forceps, grasp the edge of the blade closest to the handle and lift it up over the blade holder. Slide the blade carefully over the blade holder until it is separated from the blade handle. Do not use a lot of force, as this could cause the blade to fly out of your grasp. For large blades (brown Accu-edge) and handles. Hold the edge with the holes in the hand you will use to slide the blade. Hold it near the end that you will carefully slide into the blade housing. Reposition your fingers to further slide the blade in until you reach the other end (first hole of the blade lines with the screw opening). Screw to hand tightening. To remove the blade, unscrew until the blade is released and use the forceps to grip the hole that is exposed past the blade holder. Slowly pull the blade out from the holder and be careful not to cut yourself or anyone nearby. Ergonomics Use fatigue mats when long periods of standing are required. Place items on work station to avoid excessive bending, reaching and stretching. Use a chair if needed to avoid excessive movement. Health and Safety Manual The pathology laboratory’s health and safety manual is located online. Go to www.pathology.med.umich.edu and there is a link that will take you to the manual on the pathology homepage. ALL Pathology Department employees MUST read this health and safety manual. For further information, you can contact the Pathology Department’s safety officer, Brenda Schroeder at bschro@med.umich.edu or 734-615-7902. 10 GENERAL POLICIES 1. INTERACTION OF TEAM MEMBERS Look first to your team members if you have any questions. The order of contact is as follows: PA, Fellow, Attending, Chief Resident, Dr. Visscher or Dr. Myers. 2. ELECTRONIC CALENDARS There is an electronic calendar on the Pathology website that will tell you what service an Attending is on and also what service the Pathology Assistants are on so that you know who to page if a problem arises. You can find this calendar by going to the Pathology website and in the right upper corner click on Intranet and on the drop down menu you select calendars. There you will find all of the calendars for the Department of Pathology. These are the official calendars. 3. PLACEMENT OF REQUISITION FORMS When you are finished grossing in a specimen in Room 1, place the requisition form on the second shelf of the cart by the sign out room. The red folder is for specimens that are gross exam only. When you are going up to the 2nd floor pathology, take the forms and put them into their labeled slot across from the Front desk. A folder is present for gross exam only. When you are grossing in Room 2, take the requisition forms out by the front desk and place them in their proper labeled slot. 4. GROSS CONFERENCE Gross conference is an informal educational meeting which will be held every first Thursday of each month. Important topics will be discussed: grossing techniques, histology, differentials, etc. If you grossed in a case or know of a case which would demonstrate exceptional pathology, place specimens in designated areas (room 1- free floating cart on 2nd and 3rd shelves and room 2- Gyn cart on 2nd and 3rd shelves). Please see diagram for details. Each designated area contains a clip board containing a log in sheet as well as gross conference stickers. Make sure you log in the specific case and place a sticker on the bucket. The specimens will be collected by the PA’s (week prior to conference) and sent down to the morgue. 5. GROSS CUTTING MANUAL How to find and use it 11 The cutting manual is usually book marked in favorites on the computers at the grossing stations. It is listed either as Cutting Manual or Table of Contents. The website is http://www.pathology.med.umich.edu/Resident/Cutting_Manual/ If it isn't book marked on the computer, you can either type in the website or go to the Pathology home page. Under Intranet click on Tools and Training. After that, it opens to the Cutting Manual's main page. Just click on the specimen type you are interested in. If the computers or website are down, hard copies of the cutting manual are in room 1. They are located in the cabinets above the table saw. GENERAL POLICIES (cont) 6. GROSSING STATION CLEAN-UP PROTOCOL Use Dispatch cleaning spray, along with the SOS scrubbing sponge. All stations have their own cleaning supplies (located in the sink). Extra cleaning supplies are located under clean sinks. Pick up the cutting board and clean under it. This is a common collecting area for tissue and other debris. Rinse the station completely after being scrubbed. Each station contains a water hose on the left side. Tools should be scrubbed by hand with Dispatch and dried immediately. Tools should not be soaked; this will cause corrosion/dulling of scissors. Double check all forceps and hemostats, as tissue may reside in the teeth. No bodily fluid, tissue or ink should be left on the station or tools. All blades must be disposed of in the small sharps bin (located at each station) or in the large sharps bins (Room 1 – located under counter containing bone saw and Room 2 – located next to BE station). All inks and acetone containers should be capped and wiped clean. Remove all used swabs and dispose of in the trash. Sink should be free of debris, wiped down with Dispatch/SOS sponge and drain cleared. Run garbage disposal routinely after cleaning a colon, blood clots, and ovaries with abundant cyst fluid. All supplies/containers should be wiped down and in their respective (labeled) positions (use diagram). All completed specimens should be removed from cutting station and stored properly. Please see attached diagram of Room 1 and Room 2 for storage. Supplies* will be stocked each morning by the PA who is in on Room 1 and Derm (Room 2). *Supplies include cassette lids, swabs, sponges, paper towels, blades, ink, bags, etc. PROTOCOL FOR CONTAMINATED/UNORGANIZED STATION The last person who occupies the station should be paged immediately and asked to clean the station. If no response or actions are taken by the violator, a standard e-mail (see below) shall be sent to the chief resident, Dr. Lucas notifying them of the problem. Dr. ________________, You did not respond to our notification via page that you need to return a grossing station to a usable condition per the grossing manual. We request that, in the future, you comply with standard work policies for Gross Room organization. Please address any questions to Dr. Lucas.. This e-mail should be copied to Dr. Lucas and Lisa Taulbee. 12 Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ IMPORTANT DICTATION TIPS 1. Muscle, nerve and kidney biopsies (RK, RJ, MM, MB and IB cases) are always dictated by the techs and PA’s. 2. Speak slowly and forcefully and enunciate clearly into the microphone. 3. Avoid dictating when others are talking, laughing, or sawing, etc. 4. When dictating abbreviations specify letters, i.e. C (as in cat), D (as in day), etc. 5. When dictating body of gross start with container tissue source: "Left arm." If there is no tissue source, dictate “labeled with name and reg # only”. State the size of the container*. This will assist with container retrieval. 6. Cassette summary: 1A Tips 1B-D Remainder of specimen (ns)* ns = non saved (entirely submitted) ss = some saved 7. Words that are difficult to differentiate between: Ex. Intra/inter, hyper/hypo, etc. Need to be enunciated clearly and spelled out. 8. Dictate Frozen sections as follows: A. Dictate all the Frozen Section Gross Description (handwritten information on pink page) for all frozen section specimens in their labeled order. Dictate the cassette summary for each part but do not read the frozen report yet. All frozen cassettes are called "frozen section control" or the acronym "fsc". B. Dictate all the handwritten frozen section diagnosis for all specimen parts, which is titled "Frozen Section Report". Do them in the labeled order, dictating the part number, the diagnosis and then the initials of the Pathologist(s). C. If a frozen section was sampled from a large specimen (i.e. to check a margin) and it needs fixation, do not dictate the frozen section until the specimen is ready to be grossed; state the specimen number and that it will be dictated at a later time. 13 D. When dictating a large specimen with a frozen section, dictate the entire specimen and include the handwritten information of the Frozen Section Gross Description. List the frozen section control cassettes in the cassette summary along with the cassettes for the entire specimen (i.e. "1A - frozen section control block- ns; 1B- anterior cervix -ss, etc.) 9. Arrows cannot be typed into computer-please dictate upper, lower, increasing or decreasing instead (i.e. don’t say “arrow up”). *There are at least 6 container sizes: 1. Extra small 2. Short Small 3. Tall Small 4. Medium 5. Large 6. Extra large We will show you these containers. DIGITAL DICTATION TIPS To begin, turn the machine on (lower left corner; make sure the red light is on). If the machine does not turn on at first, remove the phone card from the back, wait 5 seconds, then plug back in. If you have a problem with the dictation system, contact Paulette Dozier at 936-6801. The volume, tone and speed levels are on the front of the unit. To make the volume louder, slide the lever to the right. Step 1: Enter your five (5) Digit Physician I.D. Everyone must be assigned an ID number before dictating; this will help identify the grosser. Note: If your I.D. is only four (4) digits, precede with a leading 0 Step 2: Touch the key corresponding with the Work Type you wish to dictate. The button marked “history/gross” is what you are about to dictate. If it is a rush specimen, press the button labeled “Rush”. Work Type Description History/Gross Diagnosis Rush Amended/Additional Consultation/Letter Autopsy M-Lab Gross/History Work Type # 1 2 3 4 5 6 8 Step 3: Touch the key corresponding with the Pre-Fix you wish to dictate Find the letter prefix on the right side of the dictation machine and press the service you are dictating (BE, IF, GU, etc.) Note: If the correct prefix is not programmed, refer to the prefixes listed below: Step 4: Please scan the requisition: Swipe the barcode label on the requisition under the barcode reader. The barcode reader is on the shelf next to the dictation machine 14 The red laser line must read the barcode in a horizontal line It will beep when the barcode information is successfully transmitted. Step 5: The system will prompt you “Ready to Dictate” The foot pedal has three sections: On the right is the pedal for record, the middle pedal is reverse and the left pedal is to listen Press the record pedal gently and speak into the microphone (black and silver adjustable wand on the shelf To stop recording, lift your foot off of the record pedal To rewind, press the reverse pedal. It will beep as it rewinds To stop rewinding, lift your foot off the pedal To listen, press the left pedal marked “listen”. You should be able to hear your voice (adjust volume as needed.) The recording function is overwrite only. It will only record not save what is there at that position of the recording, therefore, make sure you are at the end of the recording before you continue your dictation. Step 6: Begin dictation and please provide the following: Say your name and the case number (i.e. BE08-12345) Give the patient’s complete name as it is on the requisition Read all of the information from the requisition Source of specimen, history, pertinent lab data, operative procedures, tissue procurement consent and orientation, etc Step 7: After reading the information off the requisition, dictate the container label in quotations (container labeled “right breast”) Step 8: When you have completed your dictation press the Next job button on the lower right corner. Note: You will be prompted to enter the WORKTYPE, PREFIX and ACCESSION # for your next dictation (repeat steps 2, 3 and 4) If you are done with dictation and you will not be doing anymore at that time, press Exit System (lower right corner) and then turn the power off (lower left corner) Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 15 GROSS DICTATION POINTERS Account for what we received, including: Specimen container type and labeling Tissue and excision type (e.g. skin: punch biopsy vs shave biopsy, breast: core biopsy vs mastectomy) Overall dimensions Number of fragments (approximate if too many to count) with aggregate size (e.g. if sample is highly fragmented as in an endometrial curettage) Note: be very precise with mucosal and skin biopsies (i.e. “there are three bits”) Presence of orienting sutures/clips/ink Evidence of prior manipulation (i.e. post op incisions made by surgery) Use appropriate style, including: Use complete sentences when possible Correct grammar Avoid abbreviations Use descriptive, not diagnostic terms (i.e. “there is a mass” – not “there is an infiltrating ductal carcinoma”) With respect to pathology/abnormalities, describe: Distribution and size of lesion(s) Texture, color and type (e.g. mass, ulcer, cavity, discoloration…) Relationship to margins of excision and relationship to any lesions (if appropriate) 16 Presence of varied features (i.e. “the mass is partially cystic and partially solid” or “the mass if firm and tan, but there are soft areas with hemorrhage”) EXAMPLES OF GOOD GROSS DICTATION Uterus for fibroids Part 1 labeled “uterus and bilateral tubes and ovaries”. Received in formalin in a large container is a total abdominal hysterectomy specimen (8 cm from fundus to ectocervix, 5 cm from cornu to cornu and 4 cm from anterior to posterior, 120 grams) with bilateral attached adnexa. The serosal surfaces are pink, smooth and unremarkable. The ectocervical mucosa is pink, glistening and contains a patent, slit-like os. The endocervical canal is tan, glistening and contains a normal herringbone appearance. The endometrial cavity measures 4.5 cm x 3.5 cm and is lined by pink, lush and diffusely hemorrhagic endometrial mucosa. The myometrium is pink, trabeculated and remarkable for two tan, whirled, well circumscribed, intramural nodules, 0.5 cm and 2 cm. The largest nodule is remarkable for areas of necrosis and hemorrhage. The endometrium and myometrium have a greatest thickness of 0.3 and 2.5 cm. The left and right ovaries (both measure 2 x 2 x 1.5 cm) are tan and cerebriform in appearance. Sectioning of each ovary reveals pink parenchyma remarkable for multiple subcortical, smooth lined, unilocular cysts (ranging from 0.3 cm up to 0.9 cm) which contain serous fluid. The left and right fimbriated fallopian tubes (4 cm x 0.5 cm and 3.5 x 0.5 cm) are unremarkable. Cassette Summary: 1A- anterior cervix 1B-posterior cervix 1C-anterior endomyometrium 1D-posterior endomyometrium 1E- sections from each intramural nodule 1F-left ovary and tube 1G-right ovary and tube 17 (ss) Kidney for Renal Cell Carcinoma Part 2 labeled “left kidney”. Received in formalin in a large container is a radical nephrectomy specimen, 15 x 10 x 8 cm and 345 grams. The capsule (inked black) is tan, focally scarred, and strips with difficulty. The hilum contains the following: ureter (5 x 0.5 cm), renal vein (2 x 1.5 cm) and renal artery (1 x 0.4 cm). No hilar lymph nodes are identified. The specimen is bivalved to reveal a bright yellow, hemorrhagic, well circumscribed, cystic mass (4 x 4 x 3 cm) located within the calyces of the lower pole. The mass appears to abut the capsule, however does not appear to invade through it. The pelvis, renal vein and renal artery are free of invasion. The uninvolved cortex is dark brown and contains a thickness of 0.8 cm. The uninvolved medulla is tan, striated and has a width of 1 cm. The papillae are slightly blunted. The pelvis is lined by tan, glistening and unremarkable urothelium. A fragmented, unremarkable adrenal gland is identified (2 x 2 x 1 cm) within the peri-renal adipose. Pictures have been taken for reference. Cassette Summary: 1A - ureter and vascular margins (ns) 1B - mass closest to capsule 1C -1E- mass within calyces 1F - uninvolved pelvis/renal sinus 1G - uninvolved renal parenchyma 1H - adrenal EXAMPLES OF GOOD GROSS DICTATION (cont) Gallbladder Part 1 labeled “Gallbladder”. Received in formalin in a medium container is a 7.2 x 3.2 x 0.9 cm intact gallbladder. The cystic duct probes with ease. The gallbladder is partially filled with red clotted blood. No calculi are submitted with the specimen or within the container. The mucosa is tan and velvety and the wall is 0.2 cm thick. (3ss) Rectum for Cancer Part 1 labeled “Rectum and sigmoid colon”. Received in formalin in a large container is a 16.5 cm long segment of distal sigmoid and rectum. The serosa in the sigmoid is pink-tan, smooth with an abundant amount of attached adipose. The roughened attached adipose to the rectum is focally hemorrhagic with exposed areas of fibromuscular tissue. No defects are noted. The mucosa is tan and predominantly folded. The mucosa is remarkable for a 2.3 x 2.3 x 0.5 cm granular pink-tan tumor that is 2.9 cm away from the distal margin. It is 0.5 cm above the pectinate line. Tumor has a maximum depth of 0.3 cm and extends to the muscularis. It does not extend into perirectal fat. Several possible lymph nodes are identified ranging from 0.6 cm. The largest has a grey-white cystic cut surface. No other abnormalities are noted. Cassette Summary: 1A - Tumor to adjacent proximal 1B - Tumor to adjacent distal 18 1C - Tumor to deep 1D - Distal margin (ss) 1E and 1F - Multiple possible lymph nodes (ns) 1G - One bisected possible lymph node (ns) Breast Part 1 labeled “Left breast lumpectomy double stitch – deep, long stitch – lateral and short stitch superior”. Received in formalin in a medium container is a 120 gram left breast lumpectomy specimen oriented as previously mentioned, 8 (lateral and medial) x 8 (superior to inferior) x 5 cm (anterior to deep). The anterior aspect is partially surfaced by a 3.7 cm x 2.5 cm ellipse of tan skin remarkable for a 0.1 cm punctate and bluedyed probable site of previous biopsy. The specimen is inked as follows: Anterior – yellow, posterior – black, superior – blue, inferior – green, medial – red and lateral – orange. Sectioning from lateral to medial reveals a 2.5 x 1.6 x 1.5 cm pink-tan, firm, gritty and ill – defined mass that extends to within 1.0 cm of the anterior and medial margins, 1.5 cm of the deep and superior margins and 2 cm of the lateral and inferior margins. The remainder of the cut surfaces are comprised of dense fibrous tissue (85%) interspersed with adipose tissue (15%). No additional abnormalities noted. Cassette Summary: 1A-B – Mass to closest anterior margin 1C-D – Mass to closest medial margin 1E-F – Mass to closest deep margin 1G-H – Mass to closest lateral margin 1I-J – Mass to closest inferior margin 1K-L – Mass to closest superior margin EXAMPLES OF GOOD GROSS DICTATION (cont) Derm Part 1 labeled “Left arm short stitch – superior, long stitch – lateral”. Received in formalin in a small container is an ellipse of tan skin oriented as previously mentioned, 3 (superior to inferior) x 2 (lateral to medial) and excised to a depth of 1 cm. The skin surface is remarkable for a central 1 cm in diameter hypopigmented scar that extends to within 0.5 cm of the closest medial margin. The margin of the lateral half is inked blue and the medial half is inked green. Sectioning from superior to inferior reveals unremarkable cut surfaces. Cassette Summary: 1A – Superior tip 1B-D – Central portion of ellipse submitted from superior to inferior with scar in C 1E – Inferior tip (ns) Liver Part 1 Labeled with patient's name and hospital registration number. Received in formalin in a large container is a segment of liver and separately submitted gallbladder. The gallbladder is 8.7 x 4.6 x 3.2 cm. The serosa 19 is unremarkable. The fundus and body are filled with brown-yellow bile and multiple black stones that average 0.3 cm in greatest dimension. The stones crush easily. The yellow-brown mucosa is diffusely speckled with yellow areas and the cystic duct is patent. The previously sectioned liver is 1185 gm and 19.5 x 16.3 x 8.3 cm. The capsule is ragged and irregular on the posterior and inferior aspect, and there are multiple staples along the ragged margin. The anterior surface and the cut surfaces of the red-brown liver is multinodular. Within the parenchyma is a 4.5 x 3.8 x 3.2 cm, tan-grey, firm mass. This nodular mass comes to within 1.3 cm of the overlying capsule and to within 1.6 cm of the ragged surgical margin. The ragged margin is inked and the cut surface shows it to be a focally hemorrhagic, multinodular mass. No invasion into the surrounding vessels is readily seen, yet there is evidence of the tumor abutting the vascular wall, changing the shape of the lumen. Very small stellate nodules can be seen near the periphery of the main lesion. The remaining liver parenchyma is without lesions. Cassette Summary: 1A - Gallbladder 1B-C. - Tumor to inked ragged margin 1D-F - Additional sections of tumor including approximation to vascular wall (ss) 1G-H - Sections of remaining liver (ss) Colon - diverticulosis: Part 1 labeled "Sigmoid colon” Received in formalin in a large container is a 21.5 cm segment of bowel with an average diameter. There is attached mesenteric adipose tissue that is focally hemorrhagic and also focally covered with yellow-tan purulent exudate. The previously opened segment shows bowel wall of normal thickness and multiple mucosal outpouchings. Some of the outpouchings penetrate the bowel wall, pushing into the surrounding mesenteric fat. Some of the outpouchings contain brown fecal matter and/or brown-grey friable substance. Cassette Summary: 1A.-D – Outpouchings (ss) EXAMPLES OF GOOD GROSS DICTATION (cont) Bladder and Uterus Part 1 labeled "Left distal ureter” a 0.4 x 0.3 x 0.3 cm. 1A. Frozen section control blocks (ns) Part 2 labeled "Right distal ureter” a 0.4 x 0.3 x 0.3 cm. 2A. Frozen section control blocks (ns) Part 3 labeled "Urethral margin” a strip, 3.0 x 0.4 x 0.4 cm. 3A. Frozen section control block (ns) Part 4 labeled "Uterus and bladder” Received in formalin in a large container is a separately submitted bladder and uterus with cervix. The bladder with attached pericystic adipose tissue is 12.8 x 6 x 4.4 cm. The previously opened bladder has unremarkable serosa. The tan mucosal has a 2.6 x 1.5 cm slightly hemorrhagic and roughened area occupying most of the posterior wall. It extends from the trigone to the dome. This area comes to within 2.4 cm of the urethral margin, within 0.7 cm of the left ureteral orifice and within 0.6 cm of the right ureteral orifice. The remaining mucosa is slightly edematous, showing no evidence of neoplasm. The uterus and cervix is 70 grams and 7.8 x 5 x 3.4 cm. The tan-brown uterine serosa is focally adhesed from the posterior wall. The grey-purple ectocervical mucosa is partly hemorrhagic, and the endocervical mucosa is 20 yellow-tan with clear fluid-filled cystic structures up to 0.4 cm. The endocervical canal is unremarkable. The 2.6 x 2.2 cm endometrial cavity has pink-tan, hemorrhagic endometrium that averages 0.2 cm in thickness. The myometrium is 1.8 cm in maximum thickness. Cassette Summary: 4A - Frozen section control block (posterior wall) (ss) 4B - Urethral margin (ns) 4C - Bladder trigone (ss) 4D.-F - Posterior wall ragged area (ss) 4G - Left ureteral orifice (ns) 4H - Right ureteral orifice (ns) 4I - Anterior cervix (ss) 4J - Posterior cervix (ss) 4K - Anterior endomyometrium (ss) 4L - Posterior endomyometrium (ss) GENERAL INKING GUIDELINES 1. Pat the specimen dry. The ink won’t run nearly as much if the specimen is patted dry. 2. Use a cotton swab to apply ink if precision is needed. Don’t have ink dripping from the swab before applying to the specimen. If a large area is requiring ink, put some ink on gauze and dab it on the specimen. 3. Evenly cover the desired surface. 4. Use acetone as a mordant for the ink. 5. Pat the specimen dry and reapply ink to areas that it may not have stayed on. Don’t cut the specimen until it is patted dry or your ink will run onto the cut surface. 21 6. If you are using 6 ink colors, there is a template that can be used instead of always saying which margin is represented with each color. However, you must use those colors for those margins as follows: anterior – yellow, posterior – black, superior – blue, inferior – green, medial – red, and lateral – orange. 7. Red ink, when you are on dermatology, sends a message to the histotech to embed that surface down. The different ink preferences for the breast and dermatology rotations will be addressed at that time you are on those services. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ CUTTING TECHNIQUES After orientation of a specimen, section any shave margins first if needed. Place the specimen on the cutting surface and section the tissue on the short axis, unless otherwise necessary. Use a blade that is 1-1/2 to 2 times greater than the axis to be sectioned. The length of the blade should pass over the tissue without the blade edge and handle "punching” the tissue. Do not use a blade that is too short to make one slice across the entire surface. If it is too short, the tissue will appear serrated and jagged because the back of the blade and the handle is tearing the tissue. Point the tip of the blade slightly downward to reduce the surface area. Maintain the integrity of the specimen to reapproximate for further examination, if necessary. 22 If the integrity cannot be maintained after serial sectioning, arrange the remaining specimen in such a way to keep the order of sections. Use a paper towel to mark the orientation or order as a clue or reminder at the time of further exam. Sections must be no greater than 0.5 cm thickness/section on large specimens (mastectomies, liver, and lung). This will show more cut surfaces for thorough inspection. You may have to further dissect the 0.5 cm section if searching for small biopsy sites or very small nodules. If you have not visually identified pathology, palpate the 0.5 cm sections for clues. When searching for small biopsy sites/pathology, use the information in Careweb to assist in the location. When serially sectioning a specimen, section to within 0.3 cm of the cutting surface. This will leave a thin segment to help retain the integrity of the specimen. As you choose to take histology sections, use other areas to demonstrate pathology if possible. You do not have to separate the entire section from the remaining specimen. Use the forceps or your fingers to keep the tissue stable when you cut thin sections. Grasp the tissue with the tips of the forceps but do not squeeze the tissue. Try to keep a distance between the blade and your fingers of the non-cutting hand to avoid stabbing or slicing that hand/finger. Use sharp blades at all times. Cutting/stabbing injuries happen most when trying to section with a dull blade. Change your blades frequently to keep a sharp edge. When using the scissors keep the tissue near the tips and do not clog them with too much tissue. Try to avoid using scissors for the histology sections, as this may show crush artifact. Scissors may be used to trim the tissue. This should only be done after the tissue is placed in the cassette and the excess thickness can be cut with scissors to fit the lid on the cassette. With loose/mobile tissue (lung, breast) start the section with a fast, light slicing motion and once the sharp blade begins cutting through the layers then slow the slicing motion and apply more pressure on the handle toward the cutting surface. Avoid pressing the blade handle to the cutting surface until the blade has begun to cut into the tissue to minimize crushing the tissue and bending the blade handle. If the tissue needs to be laid flat on the cutting surface, put your non-cutting hand on top of the tissue with your palm flat. Apply a slight pressure to stabilize the tissue and section it horizontally. If the specimen has been inked, dry the inked surface(s) as much as possible before sectioning. Take sections for histology that will show transition of normal to tumor, tumor to an anatomic marker and/or representative tissue to an inked margin. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ COMMON TIPS FOR SPECIMEN ORIENTATION Prostate- The prostate sits in the retroperitoneal cavity and encircles the neck of the bladder/urethra. The posterior surface is flat in comparison to the anterior surface. In most DaVinci prostatectomies, the anterior aspect will be heavily studded with staples. The vas deferens and seminal vesicles are attached to the posterior aspect of the base. The apex (distal aspect) comes to a narrow point; whereas the base (proximal aspect) is wide and contains significant cauterization. 23 Bladder- The most common landmarks for bladder orientation are the ureters. Each ureter sits on the posterior/lateral aspect. The superior/posterior aspect is triangular in appearance and is covered by smooth peritoneum. The inferior aspect contains the urethra. Kidney- The most common problem for these specimens is distinguishing between superior and inferior poles. Remember, the ureter always points to the inferior pole! The renal vein (wider in diameter and thin walls) sits anterior to the ureter. The renal artery (narrow in diameter and thick/muscular walls) sits superior to renal vein and ureter. Uterus- First, use the peritoneal reflection as your guide: anterior serosal surface is higher and blunter (from bladder dissection) and posterior serosal surface is lower and comes to a point. Second, use the attached adnexa (in order of anterior to posterior): round ligament, fallopian tube, ovary and ovarian ligament. If no adnexa or cervix are present, then orientation is not always possible- state “unorientated uterus”. Colon- Orientation from proximal to distal: Cecum- blind pouch which contains appendix, largest diameter within colon. Ascending- contains terminal ileum, distal aspect has attached mesenteric adipose tissue. Hepatic Flexure- between ascending and transverse. Transverse- only segment of colon which contains omentum. Splenic Flexure- between transverse and descending. Descending- runs retroperitoneal, partially covered by peritoneum on anterior aspect. Sigmoid- lies with in pelvis and is fully covered by mesenteric adipose (sigmoid mesocolon). Rectum- covered by adventitia (not serosa), smooth longitudinal muscular layer, and no attached mesenteric adipose. Anus- contains dentate line (transition zone from colonic to anal mucosa), contains anal sphincter muscles. Lung- Left lung has 2 lobes and middle portion is called lingual. Right lung has 3 lobes. Note: right bronchus is more vertical and runs semi-parallel with trachea. Thyroid- The thyroid contains two lobes (right and left), which are connected by the isthmus. In some instances the isthmus will have a pointed pyramidal lobe which faces the superior aspect. When the pyramidal lobe is present, the thyroid will take on a W shape. The anterior capsule is smooth and convex, while the posterior capsule is flat and usually cauterized. GROSS ONLY’S EXEMPTIONS FROM MANDATORY PATHOLOGIC EXAMINATIONS 24 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. Foreign bodies Excess bone and split thickness skin grafts not used in recipient sites Scar tissue from recent burns and trauma unrelated to previous surgery for neoplastic disease Skin from cosmetic procedures Foreskins from circumcisions and plastic procedures in patients less than 6 years of age (Tokgoz et al. Int Urol Nephrol. 2004;36(4):573-6) Ribs resected in approach to retroperitoneal space and thoracotomy Urinary calculi Joint menisci Debridement for recent trauma Toenails Deviated nasal septa Medical devices and orthopedic hardware Blepharoplasty, rhytidoplasty and rhinoplasty specimens Tissue derived from removal of a fatty apron (i.e. pannus) Periesophageal fat from routine hiatal hernia repair Teeth from routine multiple extractions Grossly normal small bowel segments from bariatric procedures. Vaginal mucosa from repair operations Tympanic membrane remnants Accessory digits Tonsils and adenoids in patients less than 18 years of age unless there is a) clinical suspicion of malignancy, b) asymmetry in tonsil size (i.e. >2 times variation in greatest diameter or mass), or c) history of hematolymphoid neoplasm d) history of transplant (Erdag et al. Int J Pediatr Otorrhinolaryngol. 2005; 69:1321-5 and Sayed K et al. Pediatr and Devel Pathol. 2005; 8:533-40) Carotid endarterectomy specimens INSTRUCTIONS FOR GROSS ONLY CASES STANDARDIZED GROSS DIAGNOSIS TEMPLATE: Tonsils +/- adenoids Tonsils [and adenoids], resection: No abnormality (gross diagnosis). (t/a) Arthroplasty specimens Bone and soft tissue, R/L knee, resection: Degenerative joint disease (gross diagnosis). Bone, R/L femoral head, resection: Degenerative joint disease (gross diagnosis). (arthro) Carotid plaque Carotid plaque, endarterectomy: Atherosclerosis Rib(s) for surgical access Bone, # R/L rib, resection: No abnormality (gross diagnosis). (Ribs) Xiphoid process for post-bariatric surgery massive weight loss Bone, Xiphoid process, resection: No abnormality (gross diagnosis). 25 (Xip) Hernia sacs Soft tissue, site, resection: Hernia sac (gross diagnosis). (Hsgd) Bariatric surgeries Stomach and small bowel, resection: No abnormality (gross diagnosis). (Bari) Traumatic amputations Bone and soft tissue, site, amputation: Traumatic injury (gross diagnosis). (Bst) Abdominoplasty Skin and subcutaneous tissue, abdomen, resection: No diagnostic abnormality (gross diagnosis). (Abdo) “Foreign body” removal Body site, removal: Foreign body [or a more specific term such as ‘bullet’ where appropriate] (gross diagnosis). (Fbr) Medical/Surgical hardware removal (including implants) Body site, removal: Medical/Surgical hardware [or a more specific term such as ‘catheter’ where appropriate] (gross diagnosis). (Mshr) Varicose veins Veins, site, resection: Varicose veins (gross diagnosis). (Vvr) FEE CODES 88300 x (number of parts) • Use Number lock and G enter ID: 800 Template: or • Use F9 Phrase: enter (code) Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ COMPLETED SPECIMEN PLACEMENT 26 1. After the gross is complete on a specimen, place the container in the vented cart if there is remaining tissue (ss). If the specimen has been completely submitted (ns), put the container in the labeled red biohazard bags. **Please see diagrams of Room 1 and 2 for exact locations of carts and bags. 2. Placement of containers in vented storage carts: a. Room 1 – Specimen vented storage carts #1 and #2 are rotated at least twice a month and are taken for further storage in the morgue. Please pay attention – the carts are labeled with a start and a finish accession number. Check your accession number and place in the appropriate cart. Also, if you remove a specimen container from the cart, put it back where you got it from when you are finished. Within the cart, the containers should be placed on the shelf starting at the back, left corner and sorted according to size as follows: b. Small Short – top 2 shelves with white trays Small Tall Medium – 3rd shelf from the top Large – 4th, 5th and bottom shelves Extra Large – bottom shelf Gross Only cases should be stored in the blue containers located on the shelf underneath the bone saw area. c. Room 2 – specimen vented storage cart #1 is used to store cases with remaining tissue. Within the cart, the containers should be placed on the shelf starting at the back, left corner and sorted according to size as follows: Small Short– top 2 shelves with white trays Small Tall Medium – 2nd shelf from top Large – 4th, 5th and bottom shelves Extra large – bottom shelf Gross Only cases should be stored in the top two shelves of vented storage cart #2. If you would like to review a specimen that has been transported to the morgue call Diana French or Ricky at 6-6701 DO NOT ATTEMPT TO PULL SPECIMENS FROM THE MORGUE. NOTE: Gross specimens are discarded 30 days after sign out. When you are finished grossing and have logged your cassette blocks on the log sheet, place your cassettes in the cassette rack. Cassettes should be placed in the rack in an orderly fashion with the case being kept together as much as possible. Start at the bottom left corner to the top of the row. When that row is full; start at the bottom of the row to the right of the one that was filled. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 27 COMPLETED BLOCK PLACEMENT (cont) End Here Cassette Rack Start Here 28 HOW TO FILL OUT A SURGICAL PATHOLOGY LOG SHEET FOR CASSETTES* 1. Make sure the prefix, page #, and date are filled out. (*Labeled “A” on example logs). 2. Under part # or letter – write which part. If you have multiple cassette parts for the same accession number you can write for example 1-4. If one part has more than one cassette, 1A-C if you have 3 cassettes. (*Labeled “B” on example logs). 3. Under accession number, write the cases accession number. (*Labeled “C” on example logs). 4. Under # of cassettes – write how many you have for that part or collection of single parts. For example – 1-4 would have 4 and 1A-C would have 3. (*Labeled “D” on example logs). 5. Under # of Decal Cassettes – write the number for the cassettes that are being taken out of decal and submitted to histology. (*Labeled “E” on example logs). 6. If the case is a Rush – mark that column and in additional information write “Rush”. (*Labeled “F” on example logs). 7. The additional information has many uses. It tells histology how to handle specific cases and / or blocks. This is where you would write if you needed extra slides cut or to give slides to a specific attending. Also if the case is a rush or needs to be cut first thing in the morning, you would write “RUSH” or “1st AM CUT”. Other designations include: SQ – for derm squares; SLN for sentinel lymph nodes; B5 fixed – for B5 fixation along with the cassette designation; LBX – for Liver needle core biopsy; Scalp for alopecia punch biopsies; TUBES for fallopian tubes; LX2 for leeps or cones (*Labeled “G” on example logs). 8. Special stains forms are filled out and attached to the log sheet. 9. For Add cassettes – they are labeled different than the main specimen cassettes. The part number and that it is an Add is put on the log sheet and cassette. It then uses number 1 to how many cassettes are used. For example, if part 2 of a case needs 5 Add blocks, on the log sheet and cassettes it would be as follows: 2 ADD 1-5. (*Labeled “H” on example logs) 10. If you have a case that exceeds 26 casettes, it is lettered as AA, BB, CC NOT AB, AC, etc. *See Attached charts for visual cues 29 Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ EXAMPLE HOW TO PROPERLY FILL OUT A PATH LOG SHEET Prefix ID Room 2 *A Labels COE’D or Printed # of Specimen Jars Page 1_ Date 4/11/08 *C *D Part # or Letter *B Accession Number # of Cassettes 1A-C 2 3A-D 1 2A-D 1-2 3ADD 1-4 1-4 1A-B 2A-D 3A-Q 1E-I 16777 16677 16777 16778 16778 16779 16780 3 1 4 1 4 2 4 16781 16782 16782 16782 16783 4 2 4 17 5 Gross Only # of Blocks *E # of Blocks Slides Cut By # of Decal Cassettes H&E *F *G # of Rushes Additional Information X SLN RUSH SQ # of H&E Slides # of Frozens # of Decals # of Blanks *Explanation in “How to fill out a Surgical Pathology Log Sheet for Cassettes” document 30 EXAMPLE SURGICAL PATHOLOGY LOG SHEET Prefix______ Room______ Page______ Date______ *A *B COE Gross Room Check *C *D Part # or Letter Accession Number # of Cassettes 1A-C 12345 OR 12345 OR 12345 12345 12345 3 1-4 1A-B 2A-C 3A-C *E # of Blocks logged off Slides cut by *F # of Decal Cassettes RUSH *H Additional Information 4 2 3 3 Add Cassettes: part #/Add/# submitted (#’s ONLY. NO LETTERS 1Add2 12345 2 A=1 B=2 C=3 D=4 E=5 F=6 G=7 H=8 I=9 J=10 K=11 L=12 M=13 N=14 O=15 P=16 Q=17 R=18 S=19 T=20 U=21 V=22 W=23 X=24 Y=25 Z=26 AA=27 BB=28 CC=29 DD=30 Additional Sections: Part # / # 1 add 1 1 add 2 1 add 3 1 add 4 *H # of Specimen jars Gross Only # of Blocks # of H&E slides # of Frozens # of decals # of Blanks *Explanation in “How to fill out a Surgical Pathology Log Sheet for Cassettes” document 31 HOW TO COMPLETE IMMUNOHISTOCHEMISTRY FORM FOR SPECIAL SPECIMENS Dermatological Specimens: When you receive sentinel lymph nodes (SLN) with a dermatological specimen, you must fill out a green Anatomic Pathology I-POX form located in room 2. After filling out the top of the form with the appropriate information, check off Melan A/MART-1 (A103) and S100 protein for diagnosis of melanoma and cytokeratin 20 and cytokeratin (AE1/AE3/CAM5.2) for diagnosis of Merkel cell carcinoma. Please see attached example form for further instruction. Once you’ve filled out athe form, attach it to the back of the ID log sheet. Be sure to note SLN in the additional information column of the ID log sheet. Breast Specimens: When you receive sentinel lymph nodes (SLN) with a breast specimen, you must fill out a white Special Stain and Slide Re-cut Request form located near the BE cart. After filling out the top of the form with the appropriate information, check off Breast sentinel lymph node protocol. Please see attached example form. Once you have filled out the form, attach it to the back of the BE log sheet. Be sure to note SLN in the additional information column of the BE log sheet. Liver Transplants: When you gross a liver ex-plant case, you must fill out a white Special Stain and Slide Re-cut Request form located in Room 1. Fill out the top of the form and choose one block that contains a good representative section of liver parenchyma and write on the “Block No.” line. Next, check the Liver transplant, liver (Trichrome) line under Special Biopsy Stains. Please see attached example form. Once you have filled out the form, attach it to the back of the GA log sheet. Other Cases: There are some cases in which ordering IPOX “up front” is performed in order to expedite diagnosis. To do this: 1. On-line order entry form. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 32 DECAL PROCEDURES 1. If a specimen is calcified, hard, gritty or hard to cut – it should be placed into a decalcifying reagent. Either Cal-Ex or RDO*. 2. Stamp the requisition form with the decal code. 3. Fill out the decal log with date placed in decal, accession number, block/specimen, if you placed it in Cal-Ex or RDO. Please read Cal-Ex and RDO decalcification policies. 4. Check the decal container in the morning and in the afternoon/evening. The tissue should be soft to rubbery. You can test it with a scalpel blade. If it goes in easily then it is ready to come out of decal. Cal-Ex has formalin so it can be used on fresh or unfixed specimens. RDO doesn’t have formalin. The specimen needs to fix and then place into RDO with rinsing with water for 10 minutes in between each transfer. If the specimen isn’t fixed, you will lose nuclear detail. RDO is a stronger decal solution and specimens placed in RDO should be checked frequently. Cal-Ex has an endpoint and won’t over decalcify the specimen. 5. The sections submitted for decal must be no more than 0.3 cm thick. The cassette lids must be able to close. 6. When the specimen is ready for processing. Put the date on the cassette(s) that are taken out of decal. Place then with the regular routine cassettes checking off the decal column. NOTE: *Use Cal-Ex for femoral heads, disc, calcifications, plaque, valves, ribs, *Use RDO for bone tumor resections and skull bone DECAL LOG Date In Accn# Blocks/Specimen Cal-Ex 4-15-08 4-15-08 IF08-1111 IF-08-2222 1A 1A-E RDO X X Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 33 Date Out (to Histo) 4-16-08 4-16-08 DECAL PROCEDURE, Cont. RDO Decalcification Procedure PURPOSE: To establish a policy relative to the use of RDO to achieve decalcification of tissue in Surgical Pathology DEFINITIONS: Decalcification = the process of removing calcium from bone or tissue, thus allowing routine microtomy of paraffin embedded tissue PROCEDURE 1. Safety Considerations a. Personal Protective Equipment (PPE) should be utilized. This includes, but is not limited to gloves, protective clothing and protective eyewear. b. Do not combine formalin and RDO. Hydrochloric acid (active ingredient of RDO) and formaldehyde vapors have been reported to form a potent carcinogen, bischloromethyl ether. c. It is necessary prior to fixation in formalin is necessary. The specimen must be washed in running tap water for 10 minutes before being placed in RDO. d. Immediately after decalcification is completed the specimen must be washed in running tap water for 10 minutes then removed from RDO and placed in formalin before processing. 2. Quality Control a. Overexposure to RDO can result in poor hematoxylin staining. If this occurs, satisfactory results can be obtained by treating deparaffinized slides prior to hematoxylin with aqueous saturated lithium carbonate (1-2 minutes). b. Poor histological detail and artifacts such as swelling and fragmentation can also occur from excess decalcification. c. Hemosiderin is not removed by RDO. d. Do not use metallic equipment for decalcification as RDO corrodes most metals after long periods of exposure. 3. Storage and disposal a. Store at room temperature. Keep container closed at all times. Store only in a glass or plastic container. Do not use metal containers, as RDO will irreversibly corrode most metals. b. After long periods of storage, some change of color or an increase of suspended precipitate may occur. These are normal occurrences and do not affect the decalcifying potential of RDO. The precipitate may be allowed to settle or removed by filtration; however, neither action is necessary. c. Pour waste RDO into a properly labeled waste container. (See UMHS Classified Hazardous Waste Table in the Waste Codes binder.) 4. Specimen a. Specimen size must not exceed 2.5 cm x 2.0 cm x 0.3 cm to ensure adequate fixation and decalcification. 5. Considerations a. Do not over decalcify. Check specimen each day in the am and pm. RDO action is very rapid. b. Most specimens can be decaled in four hours or less. c. Use an adequate volume of RDO to tissue: a 20:1 volume is recommended. d. Most mature bones of 1 cm size are decalcified in 4-6 hours; smaller-cancellous bone in 2-4 hours. Bone biopsies are decalcified in 30-60 minutes. Teeth and entire femur heads may require overnight treatment. e. If RDO action is too rapid, dilute with distilled or deionized water. 34 DECAL PROCEDURE, Cont. 6. Use a. b. c. d. e. f. g. h. i. Specimen needs to be adequately fixed previous to using RDO. RDO is not a fixative. Specimen is accessioned, dictated and dissected per established procedures. Specimen is placed in a cassette(s) identified as “Decal”. Achieve appropriate level of fixation using 10% Neutral Buffered Formalin. Rinse cassette(s) in running tap water for 10 minutes. Place cassette(s) in RDO. Remove specimen from RDO when it is appropriately decalcified. Rinse cassette(s) in running tap water for 10 minutes. Place cassette(s) in container of formalin to be processed. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 35 DECAL PROCEDURE, Cont. Cal-Ex Decalcification Procedure PURPOSE: To establish a policy relative to the use of Cal-Ex to achieve decalcification of tissue in Surgical Pathology. DEFINITIONS: Cal-ex = Cal-ex is a reagent used for the simultaneous fixation and decalcification of bone and cartilage. The rapid infiltration and decalcification rate of Cal-Ex maintains cellular detail. The formic acid does not impair nuclear staining. PROCEDURE: 1. Safety Considerations a. Personal Protective Equipment (PPE) should be utilized. This includes, but is not limited to gloves, protective clothing and protective eyewear. 2. Quality Control a. Tissue may be stored in Cal-Ex for up to 2 days without adverse effects. 3. Storage and disposal a. Solutions should be stored at room temperature in a tightly closed container that is protected from direct heat and light. b. Pour waste Cal-Ex into a properly labeled waste container. (See UMHS Classified Hazardous Waste Table in the Waste Codes binder). 4. Specimen a. Specimen size must not exceed 2.5 cm x 0.3 cm to ensure adequate fixation and decalcification. 5. Considerations a. Fixation / decalcification time is dependent on the size and hardness of the calcified tissue and must be varied accordingly. Decalcification of many specimens (e.g., 3-4 mm thick blocks of vertebral bodies) is accomplished in 2-8 hours. Proportionately larger and denser bone specimens decalcify rapidly but require more time. Time should be adjusted according to practice and experience. 6. Use a. Place calcified tissue into a sufficient amount of Cal-Ex that is 5-10 times the volume of the specimen to be processed. The specimen must be completely immersed. b. After tissue is successfully decalcified it can be placed directly into 10% neutral buffered formalin and processed routinely. c. Replace old solution with fresh. Never add fresh solution to old. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 36 TABLETOP BAND SAW OPERATION Check these items before you begin: Is there water in the bottom of the reservoir? o Lift up slightly on the platform and slide it back to see into the reservoir. There must be enough to keep the sponge wet in the bottom of the reservoir under the blade. Are the 2 sponges wet? o One is in the bottom of the reservoir and the other is behind the blade on the left. Remove the sponge behind the blade and wet it. Is the blade seated in the guide that is hanging down from the motor? o If not, gently move the blade so that it is in the groove of the white plastic. Now you are ready to start the saw. To turn it on, lift the switch toward the ceiling. The switch is at the back of the motor on the top. To saw into a specimen: Start only at the bony surface. The saw does not cut through soft tissue or cartilage. If you have soft tissue covering the bone, you must cut down to the bone with a blade first. Let the saw cut through the bone in small steps. The saw will travel faster if there isn’t too much to cut all at once. Alternate the position of the bone at the blade to keep cutting it. Do not push too hard on the specimen. The motor will slow down or stop, and this means that you are pushing too hard. Wipe off excess bone sludge to keep it from splashing you. Use a C-fold towel to wipe the excess if needed. Be ready to catch the section after the blade has completed the section so that it does not fall into the reservoir. If this happens, lift the platform and retrieve it. Clean the saw with water and work station scouring sponge. Note: Do Not Attempt to cut metal with band saw. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 37 DIGITAL CAMERA OPERATION 1. Image Acquisition Open MACROPATH application Scan barcode label or enter accession number manually o Use format:IF0512345 (no dots, dashes or extra numbers) Zoom to desired level o Camera is low resolution, zoom as much as possible Click “save” pedal o Automatically sales to W:/AP_Images/gross/IMAGES/… 2. Annotation Select image to annotate in the ‘live’ window Select annotate function Perform function o Text notes o Lines o Sizing o Sections Click save or exit to live (will be prompted to save) o Automatically saves annotated image to same folder o Primary image is not altered. Note: Do Not Clean touch screen monitor with bleach or alcohol. Please use Simple Green cleaning solution that is kept under the sinks in Room 1 and Room 2. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 38 TISSUE PROCUREMENT IS TO HARVEST FRESH TISSUE FOR RESEARCH The goal of procurement is to procure tissue in the fresh state with as little time elapsing as possible. Many of the research projects are time dependant. The more time that passes the more RNA degradation occurs. If you have a fresh specimen, ask the TPC tech's if it is a case for procurement. Most large resections can be procured upon. The TPC is interested in normal, tumor and inflamed tissues. There is approximately 5-12 procurements a day. Some are quick and easy to harvest for and others may be more time consuming. When giving TPC tissue, be sure to tell them: what organ; is it tumor, normal, inflamed; accession number. Procurement is on going and dependent on when the surgical staff bring the specimens out of the OR. The tissue suitable for procurement should meet certain parameters: is there enough for diagnosis, is it viable, will it ruin the orientation. In general, tumors need to be at least 1.0 c.m. to be able to be procured upon. For normal tissue, TPC usually wants a 1.0 x 1.0 x 0.2 c.m. portion of tissue. The TPC tech's will inform you that they have a specimen for procurement and they will state how much and what they would like from the specimen. If there is an excess of tissue from what is needed for diagnosis, give them the portions asked for. If it is too small, state that and don't procure. If you can procure some but not all of what they would like, that is fine too. They usually have multiple investigators and will choose who will receive the tissue. To contact the TPC tech's if they are not in room 1 is call 48025 or 68099 or page them at 8952. If a specimen needs to be inked before sectioning, please do so before cutting into the specimen for procurement. If the weight and measurement of a specimen will be altered by procuring on the specimen, write down on the requisition form the weight and measurement prior to procuring. DO NOT submit a margin for procurement. DO NOT submit necrotic tissue for procurement. If you have an interesting case with excess tissue that isn't on the procurement list, inform TPC if they would like to procure some of the specimen before formalin is placed on it. If you have an interesting formalin fixed case, let TPC know. They have some studies that require fixed tissue. The information they need about a specimen is: accession number and medical record number. Also, what type of tissue is it and if it is normal or tumor or other. For example- IF- 1111, 1111 222 3 Thyroid T note- T is for tumor and N is for normal. After Hours Procurement If you are doing frozens and know it would be a good case for procurement, please write the accession number, what kind of tissue it is and put into a cryomold with OCT and place in the histobath in procurement. Leave a note at their station so if there are any questions they can contact you. If there is a known case coming out late and the resident is available, the TPC tech's will label and leave written and verbal instructions with the resident. If the resident will be unavailable before the tissue is submitted to Pathology, they need to inform the operating room nurse so that formalin will be placed on the specimen. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 39 LYMPHOMA The lymphoma workup is a comprehensive examination of white blood cells throughout the body, most commonly of the lymph nodes, spleen and thymus. This procedure is used to identify and differentiate from reactive and neoplastic proliferations by applying histologic examination, flow cytometry and cytogenetics. Commonly used terms: Plastic mold: polyethylene tray which is used for snap freezing Snap freeze: is a process where tissue is quickly lowered to temperatures below -70o C by placing tissue into a liquid nitrogen bath O.C.T.: resin based liquid which provides a matrix for freezing and cryostat sectioning RPMI: tissue media used for flow cytometry Procedure: Lymphoma work-ups should be performed immediately if possible. If not, place in refrigerator for no more than 1 hour. 1. Slice into section 2-3 mm think perpendicular to the long axis. 2. Fix at least one histologic section in B5 (1 hour, then formalin) and one in formalin. Fix in formalin for at least 4 hours if slides are thin. If it is a large node, sample one slice per cm for histology. If one part of the node grossly looks different (necrotic, etc.), make sure you sample that part too. 3. Use one slice for flow cytometry (at least 5 X 5 X 2 mm). Try to make sure it isn’t just fat. Mince into 1-2 mm bits and place in RPMI in a labeled plastic tube. Leave in basket in flow lab with a copy of the requisition. If the lab is closed, place in white fridge opposite the basket. 4. If additional tissue exists, snap freeze one section in plastic mold with O.C.T. Wrap in foil, label, and store in IPOX lab in -70o freezer. Leave a copy of the requisition on the door of the freezer. 5. If even more tissue is left, take a slice and put in cytogenetics media (at least 2-3 mm). Do not mince. Try to avoid any unnecessary manipulation since it will contaminate the specimen. Take specimen to Central Distribution (CD) and they will call the cytogenetics lab and arrange for pick-up. After hours, the specimen can also be taken to CD and they will keep it in their fridge for early morning pick-up. 6. Any extra (after liberal sampling) can be saved in formalin as long as it has been adequately sampled. 7. If questions arise, call the hematopathology fellow listed on the schedule with questions. Note: 1. Review the case on Careweb first. Make sure the case is in fact suspicious for lymphoma. Some biopsies come in for other diseases (i.e. sarcoma/melanoma) and only need to be put in formalin, NOT B5. 2. Dictate specimen as you normally would-Labeled, received fresh in a ____ container, # of cores and size. 3. For core biopsies: Handle cores with care. They are easily crushed. B5 has precedence over formalin, so try to submit as many cores for B5 as possible. For example, if there are 6 biopsy cores all together, submit 4 in cassette A (B5) and 2 in cassette B (formalin). Note-don’t forget to mix the premeasured B5 agents! Do not fix the designated cores in B5 for longer than 1 hour, as the tissue becomes brittle. Ancillary studies (flow cytometry and cytogenetics do not need to be performed unless specified on the requisition. 40 LYMPHOMA (cont) SPLEEN FOR LYMPHOMA Procedure: 1. Measure and weigh specimen. 2. Slice specimen as thinly as possible and examine carefully for lesions. 3. Take gross photographs of any lesions. 4. Entirely submit the hilar lymph nodes in B5 (1 hour, then formalin) and formalin. Fix for at least 4 hours. 5. See lymph node protocol for remainder of work-up to include flow cytometry, frozen tissue and cytogenetics if enough lesional tissue is present. 6. If it is too difficult to slice the spleen in thin sections, take tissue for flow and cytogenetics and then fix tissue overnight. The next day, cut thinner and examine for small lesions (ideally slices would be 2-3 mm thick). 7. Call fellow on service with any questions (schedule posted in room 1). Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 41 SINGLETON PLACENTA TEMPLATE Gross Description General Condition - say condition and use one of the options provided. Untrimmed weight: the weight of the placenta with the membranes and umbilical cord. Cord Length: give the length in centimeters Average diameter: state the average diameter Number of vessels: state the number of vessels within the cord Appearance: is there staining or shredding? If normal – pearly white Presence of true knot(s) or pseudo knot(s): if pseudo knots or true knots are present, state how many and the distance from the disk. If these are not present, say “delete knots”. Insertion: state where the umbilical cord inserts into the disk and if it is eccentric or pericentric, say how far it is from the edge of the disk. Other features: if there are any other abnormalities, state them under other features. If there is nothing to add, say delete other features. Membranes Condition: are they intact, mucoid, fragmented or other. Color: is it tan-pink, brown, green or hemorrhagic Transparency: are they opaque or translucent Membranous insertion: give the approximate % of involvement. Is it marginal? Membranes insert at the edge of the disk; circummarginate – the membranes insert near the margin with a thick fibrin rim to the margin of the disk; circumvallate – the membranes inset deep into the placenta and are surrounded by a thickened rolled rim of membranes at the insertion site. Other: state any other abnormalities or say delete others Disk Trimmed weight: the weight of the disk without the membranes or umbilical cord attached. Shape: Oval, round, bilobed-2 equal lobes, succenturiate-2 unequal lobes, accessory lobe(s) – if there is a lobe attached by blood vessels and membranes. Greatest diameter Thickness Fetal surface: color – blue grey with fibrinous deposits; green tinged, nodules; subchorionic hemorrhage. Maternal surface: adherent blood, is it intact – are there calcifications, defects Findings upon serial sectioning: hemorrhage, infract, any abnormalities Other: state if there are any other abnormalities or if not, say delete. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 42 PLACENTA (Twin) History: As for Singleton Procedure and Description If placentas are separate (nonfused), examine each placenta as a singleton. If placentas are fused, first note whether the two cords are labeled as twin A and B. If not, label them arbitrarily and make a statement to that effect. Determine the presence and type of dividing membranes. If they are absent (monochorionic-monoamniotic), so state. If they are present, remove a square of the dividing membrane, roll it, fix it for 24 hours, take a 3 mm section, and submit it for histology. Alternatively, take a “T” section. Attempt to determine grossly whether the dividing membrane has chorion or not, according to the tables on page 3. Record the kind and number of vascular anastomoses in monochorionic-diamniotic placentas: artery to artery, vein to vein, artery to vein (arteriovenous shunts). OPTIONAL: The latter can be demonstrated by injecting the artery of one twin along the plae of the fusion of the placenta with 30-50 cc of saline containing a dye (or air) and then seeing whether the fluid (or air) emerges from the vein of the other twin through one or more common villous lobules. The placenta must be intact to perform this test. Arteries always run over veins. Divide the fused twin placenta along the “vascular equator” (rather than through the base of the dividing membrane). Examine each half as a singleton placenta. Note differences in appearance of maternal surface segments for hint as to presence of twin – twin transfusion syndrome. Sections for Histology Placenta from twin A 1-2ss Membrane and cord from twin A 1 cassette Placenta from twin B 1-2ss Membranes and cord from twin B 1 cassette Dividing membrane (if present, in “T” section) 1 cassette Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 43 PLACENTA PHOTOS Getting Started: First, make sure the camera power is turned on. Click the “Med Serv” icon Click the green arrow at the top left corner Click “OK” when asked to “Start the Server” Minimize the screen Next, click the “MedMicrosc” icon Click “Viewer” at the top left corner Click “Connect” and then “Connect” again Click “File” then “New Window” Under the “New Window” heading click “grossing” Once the grossing screen pops up, change the number 0 at the top to 5 To take your first picture, and usually to clear the last pic and move on to the next, you need to click the green arrow at the top left corner Next, scan the “Begin” barcode and follow the directions on the screen. Make sure that the boarder surrounding the “Enter Value” box is blue, not gray, by clicking on it or it won’t take the pic. You can either scan the “Take Picture” barcode or click “OK” in the Enter value box to take pics You can zoom in and out using the button located at the top of the camera head Scan “End” when you are finished. Performed By: ______________________________ Date: ______________________________________ PA Observed: _______________________________ 44 HISTOLOGY PROCEDURES Histology Intro Fixation is the altering of tissue by stabilizing the protein so that it is resistant to further change. This step will determine to a large extent what can and cannot be done with the tissue subsequently. Types of fixatives include routine and special fixatives for specific cellular detail in certain tissue types. Some factors that influence fixation are: Quantity of fixative – 15 to 20 times more fixative than tissue Penetration – no fixative will penetrate more than 2-3 mm of solid tissue or 0.5 cm of porous in a 24 hour period Thickness – thickness would depend on the type of tissue, but no specimen should be more than 4 mm for a good fixation (2.5 cm x 2 cm x 3-4 mm) Processors Currently histology has five tissue processors. Small biopsies from room 2 are run routinely on processor 1 & 4. Breast and Gyn from room 2 are routinely run on processor 2. All other surgicals from room 1 are run on processor 3 along with autopsy cases. Derms are run on processor 5. Each processor is marked with its routine program number. Instructions for operation are posted in the processor room. ALWAYS CHECK WITH HISTOTECH PRIOR TO INTERUPTING A RUN. The performance at the grossing table will have a profound impact on fixation, processing and ultimately the final product. Tissue Shrinkage Tissue does not shrink as much as you might think; therefore, each specimen should be cut to the appropriate size when it is submitted into a cassette. Rubber Bands Rubber bands are not an accepted tool for securing a cassette lid. If a cassette won’t close, then the specimen needs to be trimmed down. Staples and Sutures Please always remove all sutures and staples from tissue samples before processing. They will not cut and they rip through the sections. Helpful Hints Only one piece of bone per cassette Only one piece of uterus per cassette No more than 5 serial sections of skin per cassette Never use bags when submitting derm sections 45 HISTOLOGY PROCEDURES (cont) Always take precaution when inking a specimen (especially derm) to ensure that the Tech always knows what she or he should be looking for and how to orient each sample when embedding. It is better to use multiple cassettes than to over stuff a cassette with multiple sections of tissue. 2.5 cm x 2.0 cm and no thicker than 3-4 mm is the most optimal Five Locations the Residents should know about and why: 1) Supervisor: Lisa Taulbee or Senior Clinical Technologist: Maegan Weighman. Residents should know who the responsible parties are for all areas of the Histology Laboratory. Senior Technologist make themselves available to answer questions and listen to concerns and problem solve. 2) Where to place requests for Recuts, IPOX and Special Stains Special procedures can be ordered as cases are in process or after review of slides. Expeditious receipt of requests translates to receiving stains or recuts in a more timely fashion. 3) Slide Distribution Area Case prefix will determine where completed cases are delivered to. 4) Counter Area and problem solving methodology The counter area is where the slides are labeled; cases are reconciled and delivered to the proper distribution area. The people working at the counter are in communication with residents to solve cassette labeling problems and communicate completion of rush cases. 5) Rush board in main histology lab Rush cases must be approved by an attending and communicated to the appropriate laboratory personnel. 46 Policy for Obtaining Slides and Blocks from the Slide Library PURPOSE: To establish a uniform process for the retrieval of material from the Slide Library STANDARDS: It shall be the policy of the Department of Pathology to retain, retrieve and distribute slides and blocks in a secure, confidential and timely manner in accordance with all federal specimen storage and transportation guidelines. The slide and block storage for the Department of Pathology is located in three buildings: 1. University Hospital in the UMHS 2. Medical Science 1 building 3. North Ingalls The Slide Librarian is located in the Anatomical Pathology laboratory, on the second floor of University Hospital. The Slide Librarian is responsible for overseeing the filing and retrieval of slides and blocks. To obtain material from the library a request must be submitted via the online request form (see Exhibit A). Only the Slide Librarian and/or those working with the Librarian are authorized to retrieve and distribute slides and blocks. PROCEDURE: When slides and/or blocks are needed from the library, the online form is to be filled out and submitted. The form is found on the Department of Pathology homepage under Intranet/Forms/Clinical Forms and is titled “Slide and Block Request Form”. The web address is http://www.pathology.med.umich.edu/forms/lib_req/. Only online submissions will be fulfilled. Requests are not to be made via walk-in, email, phone call, etc. Any comments/questions/concerns regarding a request can be made via phone call, email, walk-in, etc.; however, the request must still be submitted online. The form is to be filled out completely and accurately, including date needed by, time needed by and reason for request. The reasons for request are defined as follows: 1. Conference – The material requested is for a tumor board, consensus, etc. 2. Diagnosis – The material requested is needed to render a diagnosis on a case. 3. Research – The material requested is for research, not clinical purposes. 4. UMHHC Faculty/Staff – The material is being requested by a UMHHC employee for review and the material is not leaving the UMHHC system. 5. Send Out – The material requested is being sent out for review outside of the UMHHC system. The turn-around-time for the delivery of fulfilled requests is within 24 business hours for conference, diagnosis, UMHHC Faculty/Staff and Send out requests. Research requests have a two week turn-around-time, unless the request is considered large (i.e. 300 cases or more). For such requests, 100 cases will be pulled and distributed per week. During the process of pulling a request it will be documented what material is being given to the requester, and what, if any, material was not found and why it was not found (i.e. pulled for someone else, missing completely), and a copy of this will be distributed along with the material requested. Any material not found that is still determined to be needed is the responsibility of the requester to track down. The Librarian will give all information available to assist in the search. When material is no longer needed it is to be returned to the library for filing. It can be placed in any of the reading room “To be Filed” stacks, or taken to the return area outside of the Librarian’s office (room 2F337) in the Histology Laboratory in University Hospital. 47 SPECIFIC LEARNING GOALS BY SERVICE BE Inking standard Cutting fatty specimens Proper sampling Tools of the trade Breast reduction, mastectomy, biopsy and lumps Orientation of specimens Reading post mammary x-rays Implants/Cosmetic Cases Look at history for area of concern i.e. – which quadrant for disease, more than 1 mass, etc. GI Orientation Fixation When margins are important IBD, cancer, ischemic Gastric bypass, appendix, gallbladder and stromas Esophagus, whipple and distal pancreas Proper sampling of direction of sections and order of them Photography Cutting Manual GU Orientation Inking, sectioning and dictating of prostate Bladder, kidney, testicle Urethra Proper sampling GYN Orientation Inking of resections and cones Ovaries and tubes – normal vs BRCA Fallopian tubes for sterilization Vulva, cervical CA – Residents only cases Ovarian CA vs benign cysts Normal uterus vs Endo CA, hyperplasia, cervical CA POC’s Placentas Room 1 Orientation Inking Sarcoma Head, neck, and lymph nodes Heme Gross only 48 SPECIFIC LEARNING GOALS BY SERVICE (cont) Bone Use bone saw Tissue procurement Lung and thyroid Derm Squares – Don’t do it if you don’t understand Inking Proper sampling Tissue bank Sectioning – thickness Placement in cassette Scalp punches Head/neck vs body melanoma protocol IE cases Fixation Photo or diagrams SLN’s, stains (IPOX) and stains ordered prior 49 Resident Name: ________________________ Date: ________________________________ Service: ______________________________ EVALUATION EXERCISE Points Preparation Assembles appropriate clean and maintained instruments Has clean working area to minimize cross contamination Sets up dictation equipment Verifies specimen identity, cassette identity, and requisition slip identity (Patient Safety) __ __ __ __ Handling Maintains universal precautions Isolates specimen to prevent contamination Appropriately dyes or stains tissue specimen Describes number of specimens, overall external features Uses proper dissection technique to open specimen Uses proper dissection technique to obtain mandatory minimal specimen Data points (i.e. margins, nodes, precursor lesions, tumor origins) Takes mandatory minimal histologic sections, describes topography in dictation Observes all gross pathologic findings in relation to normal tissue gross appearance Takes adequate random sampling of specimen Does not take excess histologic sections Photos / Diagram Proper sectioning completely through specimen __ __ __ __ __ __ __ __ __ __ __ __ Dictation Clearly identifies the demographics and identity Clearly indicates any pertinent clinical information supplies in requisition Dictates in a slow audible and clear tone, minimizing background noise Describes the gross appearance of the specimen adequately Describes any special handling, topographic section adequately Uses commonly accepted pathology terminology to describe findings (avoids jargon) Dictates in an organized fashion Includes any OR consultation with diagnosis in dictation Notes container size __ __ __ __ __ __ __ __ __ Tissue Submission Submits appropriately labeled casettes Properly preserves all remaining tissue For special dissected residual tissue, properly wraps and identifies for future submission Properly prepares tissue for special studies, including completion of submission forms Tissue in cassette of proper size, thickness, orientation __ __ __ __ __ _______ 50 EVALUATION EXERCISE, Cont. List of Specimens: Observed Grossed COMMENTS: ____________________________________________ Signature Date 51