KS4 Extraction of Metals 1
advertisement

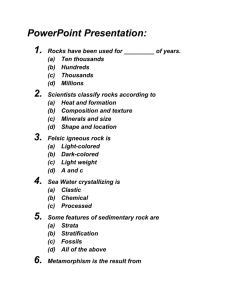
State that copper can be purified by electrolysis Describe that recycling copper is cheaper than making copper and that it saves resources Describe the use of electrolysis in the purification of copper Describe an atom as a nucleus surrounded by electrons State that a nucleus is positively charged, an electron is negatively charged, and an atom is neutral State the relative charges of electrons, protons and neutrons. Explain that that an atom is neutral since it contains the same number of protons as electrons. Describe that atoms of the noble gases (helium,neon, argon) have a stable number of electrons and that other atoms want to gain or lose electrons to achieve this number of electrons State that an ion is a charged atom or group of atoms Recognise that a particle is an ion from its formula State that positive ions are called cations and negative ions are called anions State that liquids that conduct electricity contain ions. State that the negative electrode is called the cathode and that the positive electrode is called the anode State that the three types of rock are igneous,metamorphic and sedimentary. Describe that sedimentary rocks are found in layers and that the bottom layer is most likely to the oldest layer Explain how the size of crystals in an igneous rock is related to the rate of cooling of molten rock. Explain why an igneous intrusion contains younger rock than the surrounding sedimentary rock Explain why sedimentary rocks may contain fossils and how the ages of the fossils found can indicate the age of the rock (the younger the fossils the younger the rock) Describe the structure of the Earth as a sphere with a thin rocky crust, mantle and core Describe the outer layer of the Earth as oceanic plates under oceans and continental plates forming continents Describe the lithosphere as formed of a number of large interlocking tectonic plates that can move slowly relative to each other State that the movement of tectonic plates results in volcanic activity and earthquakes at plate boundaries State that an ore is a mixture of a mineral and surrounding rock Describe that metals are extracted from ores State that a mixtures contains two or more substances that are not chemically combined Describe a compound as a pure substance that contains two or more elements chemically combined Describe an element as a substance that contains only one type of atom Identify whether a mineral is an element or a compound from its formula Describe the extraction of iron in the blast furnace Write the word equation for the reduction of iron (III) oxide by carbon and by carbon monoxide occur in the blast furnace (formulae not given) State that aluminium is extracted from its mineral using electricity Define electrolysis as the decomposition of a liquid using electricity Describe the key features of the electrolytic decomposition involved in the production of aluminium