Chemistry 30 Outcomes

Chemistry 30 Outcomes
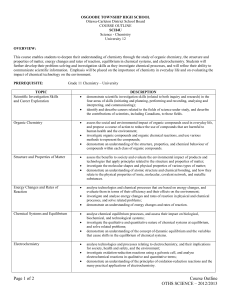
Organic Compounds
1.0
Students will identify and describe organic compounds and their chemical reactions
1.1
define organic and inorganic compounds
1.2
identify organic compounds used in daily life
1.3
name and draw organic compounds
1.4
compare properties of organic compounds based on their structure
1.5
describe processes used to separate organic compounds
1.6
define and illustrate simple addition, substitution, elimination, esterification and combustion reactions
1.7
define, illustrate and provide examples of monomers, polymers and polymerization
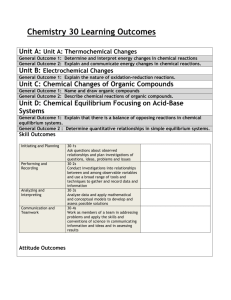
Thermochemical Changes
2.0
Students will determine and interpret energy changes in chemical reactions
2.1
Explain how energy is stored in chemical bonds
2.2
Define enthalpy and molar enthalpy
2.3
Use and interpret
H notation
2.4
Use and explain Hess’ law
2.5
Determine enthalpy changes in chemical reaction
2.6
Explain energy changes that occur during chemical reactions
2.7
Analyze and label energy diagrams of a chemical reaction
2.8
Explain the purpose of catalysts.
Electrochemical Changes
3.0
Students will explain the nature of oxidation-reduction reaction and apply the principles of oxidation-reduction to electrochemical cells.
3.1
define oxidation and reduction operationally and theoretically
3.2
compare the relative strengths of oxidizing and reducing agents
3.3
predict the spontaneity of a redox reaction
3.4
write and balance equations for redox reactions in acidic and neutral solutions
3.5
perform calculations to determine quantities of substances involved in redox titrations
3.6
identify the similarities and differences between the operation of a voltaic cell and that of an electrolytic cell
3.7
calculate the standard cell potential for electrochemical cells
3.8
calculate mass, amounts, current and time in single voltaic and electrolytic cells by applying Faraday’s law and stoichiometry.
Equilibrium in Chemical Systems
4.0
Students will explain that there is a balance of opposing reactions in chemical equilibrium systems and determine quantitative relationships in simple equilibrium systems.
4.1
define equilibrium and state the criteria that apply to a chemical system in equilibrium
4.2
identify, write and interpret chemical equations for systems at equilibrium predict, qualitatively, using Le Chatelier’s principle, shifts in equilibrium how these changes affect the equilibrium constant
4.3
define K c to predict the extent of the reaction and write equilibrium-law expressions for given chemical equations, using lowest whole-number coefficients
4.4
describe Brønsted–Lowry acids as proton donors and bases as proton acceptors
4.5
write Brønsted–Lowry equations, including indicators, and predict whether reactants or products are favoured for acid-base equilibrium reactions for monoprotic and polyprotic acids and bases
4.6
identify conjugate pairs and amphiprotic substances and buffers
4.7
define K w , K a , K b and use these to determine pH, pOH, [H
3
O
+
] and [OH – ] of acidic and basic solutions
4.8
calculate equilibrium constants and concentrations for homogeneous systems
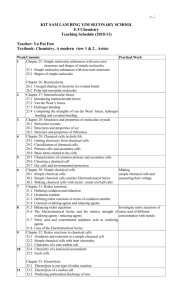
Score
Excellent
Proficient
Meeting
Criteria
The student demonstrates an excellent understanding of the outcomes and can fully and correctly supports ideas using relevant facts and details.
Statements made in the responses are organized, unambiguous, and are supported explicitly but may contain a minor error or have minor omissions.
The student demonstrates a good understanding of the
outcomes and fully and adequately supports ideas using facts and details.
Statements made in the responses are unambiguous, mostly complete, mostly correct, but may contain errors.
The student demonstrates a basic understanding of the
outcomes.
Statements made in the responses may be disorganized, ambiguous, incomplete, and may lack support.
Not meeting
The student demonstrates a limited understanding of the
outcomes.
Statements made in the response lack details, clarity, and support.