Quiz 7
advertisement
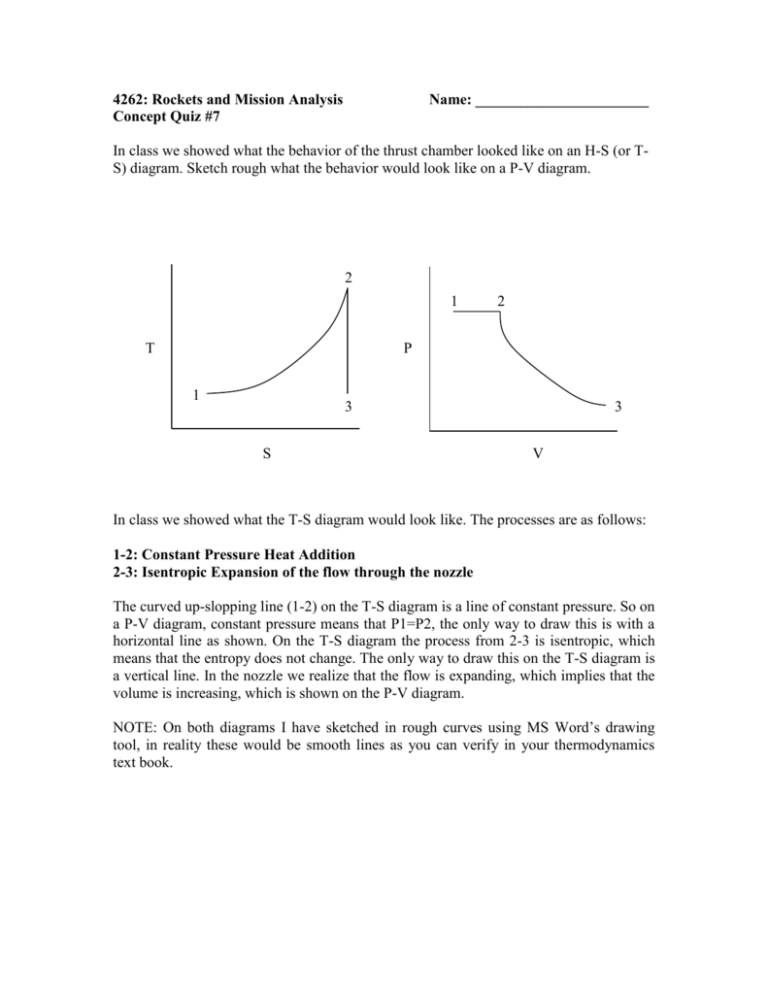
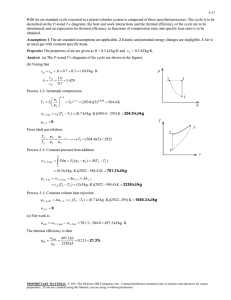
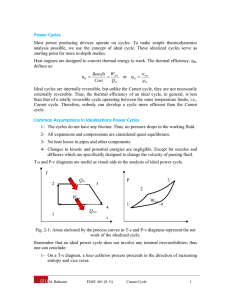
4262: Rockets and Mission Analysis Concept Quiz #7 Name: _______________________ In class we showed what the behavior of the thrust chamber looked like on an H-S (or TS) diagram. Sketch rough what the behavior would look like on a P-V diagram. 2 1 T 2 P 1 3 S 3 V In class we showed what the T-S diagram would look like. The processes are as follows: 1-2: Constant Pressure Heat Addition 2-3: Isentropic Expansion of the flow through the nozzle The curved up-slopping line (1-2) on the T-S diagram is a line of constant pressure. So on a P-V diagram, constant pressure means that P1=P2, the only way to draw this is with a horizontal line as shown. On the T-S diagram the process from 2-3 is isentropic, which means that the entropy does not change. The only way to draw this on the T-S diagram is a vertical line. In the nozzle we realize that the flow is expanding, which implies that the volume is increasing, which is shown on the P-V diagram. NOTE: On both diagrams I have sketched in rough curves using MS Word’s drawing tool, in reality these would be smooth lines as you can verify in your thermodynamics text book.